The role of DPYD and the effects of DPYD suppressor luteolin combined with 5-FU in pancreatic cancer
- PMID: 39158384
- PMCID: PMC11331593
- DOI: 10.1002/cam4.70124
The role of DPYD and the effects of DPYD suppressor luteolin combined with 5-FU in pancreatic cancer
Abstract
Background: Despite advances in the treatment of cancer, pancreatic ductal adenocarcinoma (PDAC) remains highly lethal due to the lack of effective therapies. Our previous study showed that Luteolin (Lut), a flavonoid, suppressed pancreatocarcinogenesis and reduced the expression of dihydropyrimidine dehydrogenase (DPYD), an enzyme that degrades pyrimidines such as 5-fluorouracil (5-FU), in PDACs. In this study, we investigated the role of DPYD and evaluated the therapeutic potential of combining 5-FU with Lut in PDACs.
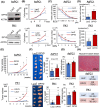
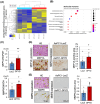
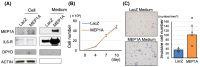
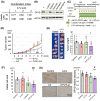
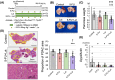
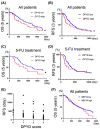
Methods and results: PDAC cells overexpressing DPYD showed increased proliferation, and invasiveness, adding to the resistance to 5-FU. The xenograft tumors of DPYD-overexpressing PDAC cells also exhibit enhanced growth and invasion compared to the control xenograft tumors. RNA-seq analysis of the DPYD-overexpressing PDAC xenograft tumors revealed an upregulation of genes associated with metallopeptidase activity-MMP9 and MEP1A. Furthermore, the overexpression of MEP1A in PDAC was associated with invasion. Next, we investigated the combined effects of Lut, a DPYD suppressor, and 5-FU on DPYD-overexpressing xenograft tumors and PDAC of Pdx1-Cre; LSL-KrasG12D/+; Trp53flox/flox(KPPC) mice. Neither single administration of 5-FU nor Lut showed significant inhibitory effects; however, the combined administration of 5-FU and Lut exhibited a significant tumor-suppressive effect in both the xenograft tumors and KPPC models.
Conclusion: We have elucidated that DPYD expression contributes to proliferation, invasiveness, and 5-FU resistance, in PDACs. The combination therapy of Lut and 5-FU holds the potential for enhanced efficacy against PDACs.
Keywords: 5‐fluorouracil; combination drug therapy; dihydropyrimidine dehydrogenase; luteolin; pancreatic cancer.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no potential conflicts of interest to declare.
Figures
Similar articles
-
DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer.Carcinogenesis. 2021 Jul 16;42(7):940-950. doi: 10.1093/carcin/bgab017. Carcinogenesis. 2021. PMID: 33640964 Free PMC article.
-
Cancer-Specific Targeting of Taurine-Upregulated Gene 1 Enhances the Effects of Chemotherapy in Pancreatic Cancer.Cancer Res. 2021 Apr 1;81(7):1654-1666. doi: 10.1158/0008-5472.CAN-20-3021. Epub 2021 Mar 1. Cancer Res. 2021. PMID: 33648930
-
Oncogenic GALNT5 confers FOLFIRINOX resistance via activating the MYH9/ NOTCH/ DDR axis in pancreatic ductal adenocarcinoma.Cell Death Dis. 2024 Oct 21;15(10):767. doi: 10.1038/s41419-024-07110-w. Cell Death Dis. 2024. PMID: 39433745 Free PMC article.
-
Clinical implications of dihydropyrimidine dehydrogenase (DPD) activity in 5-FU-based chemotherapy: mutations in the DPD gene, and DPD inhibitory fluoropyrimidines.Int J Clin Oncol. 2003 Jun;8(3):132-8. doi: 10.1007/s10147-003-0330-z. Int J Clin Oncol. 2003. PMID: 12851836 Review.
-
Dihydropyrimidine dehydrogenase in the metabolism of the anticancer drugs.Cancer Chemother Pharmacol. 2019 Dec;84(6):1157-1166. doi: 10.1007/s00280-019-03936-w. Epub 2019 Sep 4. Cancer Chemother Pharmacol. 2019. PMID: 31482228 Review.
Cited by
-
Exploring experimental models of prostate cancer in chemoprevention: Oxidative stress as a key pathway to translational research.Pathol Int. 2025 Mar;75(3):131-144. doi: 10.1111/pin.13509. Epub 2025 Jan 14. Pathol Int. 2025. PMID: 39807695 Free PMC article. Review.
-
Unlocking the Potential of Bioactive Compounds in Pancreatic Cancer Therapy: A Promising Frontier.Biomolecules. 2025 May 15;15(5):725. doi: 10.3390/biom15050725. Biomolecules. 2025. PMID: 40427617 Free PMC article. Review.
References
-
- American Cancer Society . Cancer Facts & Figures 2023. American Cancer Society; 2023:21.
-
- Cancer Statistics . Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan, Ministry of Health, Labour and Welfare).
-
- Pandurangan AK, Esa NM. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev. 2014;15:5501‐5508. - PubMed
-
- Sagawa H, Naiki‐Ito A, Kato H, et al. Connexin 32 and luteolin play protective roles in non‐alcoholic steatohepatitis development and its related hepatocarcinogenesis in rats. Carcinogenesis. 2015;36:1539‐1549. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

