Ultrafast Cooling With Total Liquid Ventilation Mitigates Early Inflammatory Response and Offers Neuroprotection in a Porcine Model of Cardiac Arrest
- PMID: 39158568
- PMCID: PMC11963922
- DOI: 10.1161/JAHA.124.035617
Ultrafast Cooling With Total Liquid Ventilation Mitigates Early Inflammatory Response and Offers Neuroprotection in a Porcine Model of Cardiac Arrest
Abstract
Background: Brain injury is one of the most serious complications after cardiac arrest (CA). To prevent this phenomenon, rapid cooling with total liquid ventilation (TLV) has been proposed in small animal models of CA (rabbits and piglets). Here, we aimed to determine whether hypothermic TLV can also offer neuroprotection and mitigate cerebral inflammatory response in large animals.
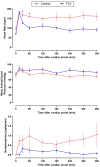
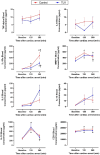
Methods and results: Anesthetized pigs were subjected to 14 minutes of ventricular fibrillation followed by cardiopulmonary resuscitation. After return of spontaneous circulation, animals were randomly subjected to normothermia (control group, n=8) or ultrafast cooling with TLV (TLV group, n=8). In the latter group, TLV was initiated within a window of 15 minutes after return of spontaneous circulation and allowed to reduce tympanic, esophageal, and bladder temperature to the 32 to 34 °C range within 30 minutes. After 45 minutes of TLV, gas ventilation was resumed, and hypothermia was maintained externally until 3 hours after CA, before rewarming using heat pads (0.5 °C-1 °C/h). After an additional period of progressive rewarming for 3 hours, animals were euthanized for brain withdrawal and histological analysis. At the end of the follow-up (ie, 6 hours after CA), histology showed reduced brain injury as witnessed by the reduced number of Fluroro-Jade C-positive cerebral degenerating neurons in TLV versus control. IL (interleukin)-1ra and IL-8 levels were also significantly reduced in the cerebrospinal fluid in TLV versus control along with cerebral infiltration by CD3+ cells. Conversely, circulating levels of cytokines were not different among groups, suggesting a discrepancy between local and systemic inflammatory levels.
Conclusions: Ultrafast cooling with TLV mitigates neuroinflammation and attenuates acute brain lesions in the early phase following resuscitation in large animals subjected to CA.
Keywords: cardiac arrest; hypothermia; inflammation; liquid ventilation.
Figures




References
-
- Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Rsb C, Geocadin RG, Jauch EC, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017 - DOI - PubMed
-
- Chenoune M, Lidouren F, Adam C, Pons S, Darbera L, Bruneval P, Ghaleh B, Zini R, Dubois‐Randé J‐L, Carli P, et al. Ultrafast and whole‐body cooling with total liquid ventilation induces favorable neurological and cardiac outcomes after cardiac arrest in rabbits. Circulation. 2011;124:901–911. doi: 10.1161/CIRCULATIONAHA.111.039388 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

