Trends in adoption of extravascular cardiac implantable electronic devices: the Dutch cohort
- PMID: 39158682
- PMCID: PMC11413308
- DOI: 10.1007/s12471-024-01892-6
Trends in adoption of extravascular cardiac implantable electronic devices: the Dutch cohort
Abstract
Introduction: Conventional implantable cardioverter-defibrillators (ICDs) and pacemakers carry a risk of pocket- and lead-related complications in particular. To avoid these complications, extravascular devices (EVDs) have been developed, such as the subcutaneous ICD (S-ICD) and leadless pacemaker (LP). However, data on patient or centre characteristics related to the actual adoption of EVDs are lacking.
Objective: To assess real-world nationwide trends in EVD adoption in the Netherlands.
Methods: Using the Netherlands Heart Registration, all consecutive patients with a de novo S‑ICD or conventional single-chamber ICD implantation between 2012-2020, or de novo LP or conventional single-chamber pacemaker implantation between 2014-2020 were included. Trends in adoption are described for various patient and centre characteristics.
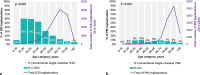
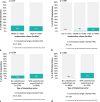
Result: From 2012-2020, 2190 S‑ICDs and 10,683 conventional ICDs were implanted; from 2014-2020, 712 LPs and 11,103 conventional pacemakers were implanted. The general use has increased (S-ICDs 8 to 21%; LPs 1 to 8%), but this increase seems to have reached a plateau. S‑ICD recipients were younger than conventional ICD recipients (p < 0.001) and more often female (p < 0.001); LP recipients were younger than conventional pacemaker recipients (p < 0.001) and more often male (p = 0.03). Both S‑ICDs and LPs were mainly implanted in high-volume centres with cardiothoracic surgery on-site, although over time S‑ICDs were increasingly implanted in centres without cardiothoracic surgery (p < 0.001).
Conclusion: This nationwide study demonstrated a relatively quick adoption of innovative EVDs with a plateau after approximately 4 years. S‑ICD use is especially high in younger patients. EVDs are mainly implanted in high-volume centres with cardiothoracic surgery back-up, but S‑ICD use is expanding beyond those centres.
Keywords: Adoption; Extravascular; Leadless pacemaker; Registration; Subcutaneous ICD.
© 2024. The Author(s).
Conflict of interest statement
K.T.N. Breeman received an unrestricted research grant from Medtronic. R.E. Knops received consulting honoraria from Abbott, Boston Scientific, Medtronic, and Cairdac and has stock options from AtaCor Medical Inc. L.V.A. Boersma received consulting honoraria, paid to his institution, from Medtronic, Boston Scientific, Abbott, Philips and Acutus, has served on a steering committee for the EMPOWER and EV-ICD trials and as an editor for the Netherlands Heart Journal. S.-C. Yap received institutional research grants and honoraria from Boston Scientific, Medtronic and Biotronik. A.H. Maass received consulting honoraria, paid to his institution, from Boston Scientific and Medtronic. F.V.Y. Tjong F.V.Y. Tjong received consulting honoraria from Abbott and Boston Scientific, paid to her institution (no personal financial gain) and support from the Dutch Research Council (NWO Rubicon grant number 452019308) and Amsterdam Cardiovascular Sciences. M.D. van der Stoel, L. van Erven, V.F. van Dijk and A.A.M. Wilde declare that they have no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources

