Performance of a Novel Continuous Glucose Monitoring Device in People With Diabetes
- PMID: 39158986
- PMCID: PMC11418503
- DOI: 10.1177/19322968241267774
Performance of a Novel Continuous Glucose Monitoring Device in People With Diabetes
Abstract
Background: In this multicenter study, performance of a novel continuous glucose monitoring (CGM) system was evaluated.
Methods: Adult participants with diabetes were included in the study. They each wore three sensors of the CGM system on the upper arms for up to 14 days. During four in-clinic visits, frequent comparison measurements with capillary blood glucose (BG) samples were performed. The primary endpoint was the 20/20 agreement rate (AR): the percentage of CGM readings within ±20 mg/dL (at BG values <100 mg/dL) or ±20% (at BG values ≥100 mg/dL) of the comparator. Further evaluations included mean absolute relative difference (MARD) and 20/20 AR in different BG ranges and across the wear time.
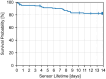
Results: Data from 48 participants and 139 sensors were analyzed. During in-clinic sessions the 20/20 AR was 90.5% and the MARD was 9.2%. For BG ranges <70, 70-180, and >180 mg/dL, 20/20 AR was 94.3%, 89.0%, and 92.5%, respectively. At the beginning, middle, and end of sensor wear time, 20/20 AR was 92.8%, 91.5%, and 85.9%, respectively. The 14-day survival probability was 82.4%. Pain and bleeding after sensor insertion were within the expected range. Based on the study outcome, the use of the device is regarded as safe.
Conclusions: The system showed a good performance compared to capillary BG measurements. This level of accuracy could be shown over the entire measurement range, especially in the low glycemic range, and the whole wear time of the sensors. The results of this study are supporting a non-adjunctive use of the device.
Keywords: MARD; accuracy; continuous glucose monitoring; performance.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JKM is a member in the advisory boards of Abbott Diabetes Care, Becton-Dickinson/Embecta, Biomea, Eli Lilly, Medtronic, Novo Nordisk, PharmaSens, Roche Diabetes Care, Sanofi, and Viatris; received speaker honoraria from Abbott Diabetes Care, A. Menarini Diagnostics, Becton-Dickinson/Embecta, Boehringer-Ingelheim, Eli Lilly, MedTrust, Novo Nordisk, Roche Diabetes Care, Sanofi, Servier, and Ypsomed; and is shareholder of decide Clinical Software GmbH and elyte Diagnostics GmbH. GF is general manager and medical director of the IfDT (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies, eg, with medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IfDT have received speakers’ honoraria or consulting fees in the last three years from Abbott, Berlin Chemie, Boydsense, Dexcom, Lilly Deutschland, Novo Nordisk, Perfood, PharmaSens, Roche, Sinocare, Terumo, and Ypsomed. ME and DW are employees of IfDT. WM-H, KM, MA, GV, and CCR are employees of Roche Diabetes Care GmbH, Mannheim, Germany. TF is Chief Medical Officer and Chief Operations Officer of Clinical Research Services, Mannheim, Germany. TF received speaker honoraria or consulting fees in the last three years from Amarin, Astra Zeneca, Bayer, Boehringer-Ingelheim, Cipla, Daiichi-Sankyo, derCampus, Diabetes Academy Bad Mergentheim, Eli Lilly, Fortbildungskolleg, MSD, Novo Nordisk, Roche Diagnostics, Sanofi, Santis, and Sciarc. TF serves as associate editor for Endocrinology, Diabetes & Metabolism.
Figures




References
-
- Clinical Laboratory Standards Institute. POCT05—Performance metrics for continuous interstitial glucose monitoring. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

