From Cell to Gene: Deciphering the Mechanism of Heart Failure With Single-Cell Sequencing
- PMID: 39159065
- PMCID: PMC11497092
- DOI: 10.1002/advs.202308900
From Cell to Gene: Deciphering the Mechanism of Heart Failure With Single-Cell Sequencing
Abstract
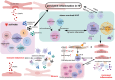
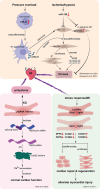
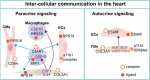
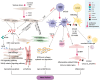
Heart failure (HF) is a prevalent cardiovascular disease with significant morbidity and mortality rates worldwide. Due to the intricate structure of the heart, diverse cell types, and the complex pathogenesis of HF, further in-depth investigation into the underlying mechanisms is required. The elucidation of the heterogeneity of cardiomyocytes and the intercellular communication network is particularly important. Traditional high-throughput sequencing methods provide an average measure of gene expression, failing to capture the "heterogeneity" between cells and impacting the accuracy of gene function knowledge. In contrast, single-cell sequencing techniques allow for the amplification of the entire genome or transcriptome at the individual cell level, facilitating the examination of gene structure and expression with unparalleled precision. This approach offers valuable insights into disease mechanisms, enabling the identification of changes in cellular components and gene expressions during hypertrophy associated with HF. Moreover, it reveals distinct cell populations and their unique roles in the HF microenvironment, providing a comprehensive understanding of the cellular landscape that underpins HF pathogenesis. This review focuses on the insights provided by single-cell sequencing techniques into the mechanisms underlying HF and discusses the challenges encountered in current cardiovascular research.
Keywords: application; challenging; heart failure; mechanism; single‐cell sequencing.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tang F., Barbacioru C., Wang Y., Nordman E., Lee C., Xu N., Wang X., Bodeau J., Tuch B. B., Siddiqui A., Lao K., Surani M. A., Nat. Methods 2009, 6, 377. - PubMed
-
- Ishii S., Tago K., Senoo K., Appl. Microbiol. Biotechnol. 2010, 86, 1281. - PubMed
-
- Zhu Z., Chen X., Genom. Appl. Biol. 2015, 34, 902.
-
- Yan D., C. Song, He H., Chem. Indust. Engineer. 2015, 32, 71.
-
- Lu Z., Moraes C., Zhao Y., You L., Simmons C. A., Sun Y., in IEEE International Conference on Robotics and Automation 2010, 494.
Publication types
MeSH terms
Grants and funding
- 2023ZYD0093/Central Government Guides Local Science and Technology Development Fund Project
- 2023RCX174/Luzhou Science and Technology Bureau
- CMEMR2017-B08/Guangxi Normal University
- 221YYJC1944/Sichuan Province Science and Technology Support Program
- 2022YFS0607/Sichuan Province Science and Technology Support Program
- 2022YFS0615/Sichuan Province Science and Technology Support Program
- 81700308/National Natural Science Foundation of China
- 81900300/National Natural Science Foundation of China
- 82301658/National Natural Science Foundation of China
- 2021LZXNYD-Z05/Joint Funds of Southwest Medical University and Luzhou Government
- Grants from Southwest Medical University
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
