Obesity and the gut microbiota: implications of neuroendocrine and immune signaling
- PMID: 39159270
- PMCID: PMC11927058
- DOI: 10.1111/febs.17249
Obesity and the gut microbiota: implications of neuroendocrine and immune signaling
Abstract
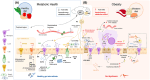
Obesity is a major health challenge due to its high prevalence and associated comorbidities. The excessive intake of a diet rich in fat and sugars leads to a persistent imbalance between energy intake and energy expenditure, which increases adiposity. Here, we provide an update on relevant diet-microbe-host interactions contributing to or protecting from obesity. In particular, we focus on how unhealthy diets shape the gut microbiota and thus impact crucial intestinal neuroendocrine and immune system functions. We describe how these interactions promote dysfunction in gut-to-brain neuroendocrine pathways involved in food intake control and postprandial metabolism and elevate the intestinal proinflammatory tone, promoting obesity and metabolic complications. In addition, we provide examples of how this knowledge may inspire microbiome-based interventions, such as fecal microbiota transplants, probiotics, and biotherapeutics, to effectively combat obesity-related disorders. We also discuss the current limitations and gaps in knowledge of gut microbiota research in obesity.
Keywords: diet; enteroendocrine hormones; gut microbiota; inflammation; obesity.
© 2024 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
Romaní‐Pérez M and Sanz Y are co‐inventors of probiotic patents for obesity. Other authors declare no competing interests.
Figures


References
-
- (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894, i–xii, 1–253. - PubMed
-
- Valenzuela PL, Carrera‐Bastos P, Castillo‐García A, Lieberman DE, Santos‐Lozano A & Lucia A (2023) Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol 20, 475–494. - PubMed
-
- van Galen KA, Schrantee A, Ter Horst KW, la Fleur SE, Booij J, Constable RT, Schwartz GJ, DiLeone RJ & Serlie MJ (2023) Brain responses to nutrients are severely impaired and not reversed by weight loss in humans with obesity: a randomized crossover study. Nat Metab 5, 1059–1072. - PubMed
-
- Duca FA, Swartz TD, Sakar Y & Covasa M (2013) Decreased intestinal nutrient response in diet‐induced obese rats: role of gut peptides and nutrient receptors. Int J Obes 37, 375–381. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

