RNase-mediated reprogramming of Yersinia virulence
- PMID: 39159284
- PMCID: PMC11361751
- DOI: 10.1371/journal.ppat.1011965
RNase-mediated reprogramming of Yersinia virulence
Abstract
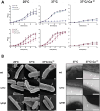
RNA degradation is an essential process that allows bacteria to regulate gene expression and has emerged as an important mechanism for controlling virulence. However, the individual contributions of RNases in this process are mostly unknown. Here, we tested the influence of 11 potential RNases in the intestinal pathogen Yersinia pseudotuberculosis on the expression of its type III secretion system (T3SS) and associated effectors (Yops) that are encoded on the Yersinia virulence plasmid. We found that exoribonuclease PNPase and endoribonuclease RNase III inhibit T3SS and yop gene transcription by repressing the synthesis of LcrF, the master activator of Yop-T3SS. Loss of both RNases led to an increase in lcrF mRNA levels. Our work indicates that PNPase exerts its influence via YopD, which accelerates lcrF mRNA degradation. Loss of RNase III, on the other hand, results in the downregulation of the CsrB and CsrC RNAs, thereby increasing the availability of active CsrA, which has been shown previously to enhance lcrF mRNA translation and stability. This CsrA-promoted increase of lcrF mRNA translation could be supported by other factors promoting the protein translation efficiency (e.g. IF-3, RimM, RsmG) that were also found to be repressed by RNase III. Transcriptomic profiling further revealed that Ysc-T3SS-mediated Yop secretion leads to global reprogramming of the Yersinia transcriptome with a massive shift of the expression from chromosomal to virulence plasmid-encoded genes. A similar reprogramming was also observed in the RNase III-deficient mutant under non-secretion conditions. Overall, our work revealed a complex control system where RNases orchestrate the expression of the T3SS/Yop machinery on multiple levels to antagonize phagocytic uptake and elimination by innate immune cells.
Copyright: © 2024 Meyer et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

