Ginsenoside Rg3 Restores Mitochondrial Cardiolipin Homeostasis via GRB2 to Prevent Parkinson's Disease
- PMID: 39159293
- PMCID: PMC11497058
- DOI: 10.1002/advs.202403058
Ginsenoside Rg3 Restores Mitochondrial Cardiolipin Homeostasis via GRB2 to Prevent Parkinson's Disease
Abstract
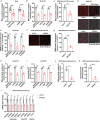
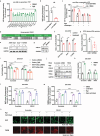
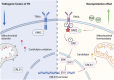
Regulating cardiolipin to maintain mitochondrial homeostasis is a promising strategy for addressing Parkinson's disease (PD). Through a comprehensive screening and validation process involving multiple models, ginsenoside Rg3 (Rg3) as a compound capable of enhancing cardiolipin levels is identified. This augmentation in cardiolipin levels fosters mitochondrial homeostasis by bolstering mitochondrial unfolded protein response, promoting mitophagy, and enhancing mitochondrial oxidative phosphorylation. Consequently, this cascade enhances the survival of tyrosine hydroxylase positive (TH+) dopaminergic neurons, leading to an amelioration in motor performance within PD mouse models. Using limited proteolysis-small-molecule mapping combined with molecular docking analysis, it has confirmed Growth Factor Receptor-Bound Protein 2 (GRB2) as a molecular target for Rg3. Furthermore, these investigations reveal that Rg3 facilitates the interaction between GRB2 and TRKA (Neurotrophic Tyrosine Kinase, Receptor, Type 1), thus promotes EVI1 (Ecotropic Virus Integration Site 1 Protein Homolog) phosphorylation by ERK, subsequently increases CRLS1 (Cardiolipin Synthase 1) gene expression and boosts cardiolipin synthesis. The absence of GRB2 or CRLS1 significantly attenuates the beneficial effects of Rg3 on PD symptoms. Finally, Tenofovir Disoproxil Fumarate (TDF) that also promotes the binding between GRB2 and TRKA is further identified. The identified compounds, Rg3 and TDF, exhibit promising potential for the prevention of PD by bolstering cardiolipin expression and reinstating mitochondrial homeostasis.
Keywords: CRLS1; EVI1; GRB2; TRKA; cardiolipin.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kalia L. V., Lang A. E., The Lancet 2015, 386, 896. - PubMed
-
- a) Kim S., Wong Y. C., Gao F., Krainc D., Nat. Commun. 2021, 12, 1807; - PMC - PubMed
- b) Senyilmaz D., Virtue S., Xu X., Tan C. Y., Griffin J. L., Miller A. K., Vidal‐Puig A., Teleman A. A., Nature 2015, 525, 124; - PMC - PubMed
- c) Mor D. E., Sohrabi S., Kaletsky R., Keyes W., Tartici A., Kalia V., Miller G. W., Murphy C. T., Proc. Natl. Acad. Sci. USA 2020, 117, 26438. - PMC - PubMed
-
- a) Choi M. L., Chappard A., Singh B. P., Maclachlan C., Rodrigues M., Fedotova E. I., Berezhnov A. V., De S., Peddie C. J., Athauda D., Virdi G. S., Zhang W., Evans J. R., Wernick A. I., Zanjani Z. S., Angelova P. R., Esteras N., Vinokurov A. Y., Morris K., Jeacock K., Tosatto L., Little D., Gissen P., Clarke D. J., Kunath T., Collinson L., Klenerman D., Abramov A. Y., Horrocks M. H., Gandhi S., Nat. Neurosci. 2022, 25, 1134; - PMC - PubMed
- b) Di Maio R., Barrett P. J., Hoffman E. K., Barrett C. W., Zharikov A., Borah A., Hu X., McCoy J., Chu C. T., Burton E. A., Hastings T. G., Greenamyre J. T., Sci. Transl. Med. 2016, 8, 342ra78; - PMC - PubMed
- c) Tanudjojo B., Shaikh S. S., Fenyi A., Bousset L., Agarwal D., Marsh J., Zois C., Heman‐Ackah S., Fischer R., Sims D., Melki R., Tofaris G. K., Nat. Commun. 2021, 12, 3817. - PMC - PubMed
-
- a) Schildknecht S., Di Monte D. A., Pape R., Tieu K., Leist M., Trends Pharmacol. Sci. 2017, 38, 541; - PubMed
- b) Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., Comyns K., Richards M. B., Meng C., Priestley B., Fernandez H. H., Cambi F., Umbach D. M., Blair A., Sandler D. P., Langston J. W., Environ. Health Perspect. 2011, 119, 866. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
