Improving normothermic machine perfusion and blood transfusion through biocompatible blood silicification
- PMID: 39159377
- PMCID: PMC11363281
- DOI: 10.1073/pnas.2322418121
Improving normothermic machine perfusion and blood transfusion through biocompatible blood silicification
Abstract
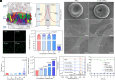
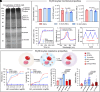
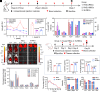
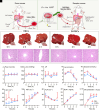
The growing world population and increasing life expectancy are driving the need to improve the quality of blood transfusion, organ transplantation, and preservation. Here, to improve the ability of red blood cells (RBCs) for normothermic machine perfusion, a biocompatible blood silicification approach termed "shielding-augmenting RBC-in-nanoscale amorphous silica (SARNAS)" has been developed. The key to RBC surface engineering and structure augmentation is the precise control of the hydrolysis form of silicic acid to realize stabilization of RBC within conformal nanoscale silica-based exoskeletons. The formed silicified RBCs (Si-RBCs) maintain membrane/structural integrity, normal cellular functions (e.g., metabolism, oxygen-carrying capability), and enhance resistance to external stressors as well as tunable mechanical properties, resulting in nearly 100% RBC cryoprotection. In vivo experiments confirm their excellent biocompatibility. By shielding RBC surface antigens, the Si-RBCs provide universal blood compatibility, the ability for allogeneic mechanical perfusion, and more importantly, the possibility for cross-species transfusion. Being simple, reliable, and easily scalable, the SARNAS strategy holds great promise to revolutionize the use of engineered blood for future clinical applications.
Keywords: antigen blocking; normothermic machine perfusion; red blood cell; silicification.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Guarrera J. V., et al. , Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transplant. 10, 372–381 (2010). - PubMed
-
- Thompson E. R., Connelly C., Ali S., Sheerin N. S., Wilson C. H., Cell therapy during machine perfusion. Transpl. Int. 34, 49–58 (2021). - PubMed
-
- Nasralla D., et al. , A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- 21972047 52003086 12072318/National Natural Science Foundation of China
- 2019QN01Y314/| Guangdong Provincial Pearl River Talents Program ()
- 2019ZT08Y318/the Program for Guangdong Introducing Innovative and Entrepreneurial Teams
- 2021A1515010724/Natural Science Foundation of Guangdong Province, China
- 202102020352 202102020259/Science and Technology Project of Guangzhou, China
LinkOut - more resources
Full Text Sources

