Evidence-Based Clinical Guidelines for Chronic Constipation 2023
- PMID: 39159626
- PMCID: PMC11825134
- DOI: 10.1159/000540912
Evidence-Based Clinical Guidelines for Chronic Constipation 2023
Abstract
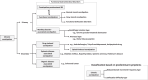
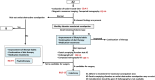
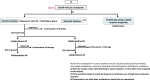
The Japan Gastroenterological Association published the first version of its clinical guidelines for chronic constipation 2023. Based on the latest evidence, these guidelines describe the definition, classification, diagnostic criteria, diagnostic testing methods, epidemiology, pathophysiology, and treatment of chronic constipation. They include flowcharts for both diagnosis and treatment of chronic constipation. In the treatment of chronic constipation, the first step involves differentiating between secondary forms, such as organic disease-associated constipation, systemic disease-associated constipation, and drug-induced constipation. The next step is to determine whether the chronic constipation stems from a motility disorder, a form of primary chronic constipation. For functional constipation and constipation-predominant irritable bowel syndrome, treatment should be initiated after evaluating symptoms like reduced bowel movement frequency type or defecation difficulty type. The first line of treatment includes the improvement of lifestyle habits and diet therapy. The first drugs to consider for oral treatment are osmotic laxatives. If these are ineffective, secretagogues and ileal bile acid transporter inhibitors are candidates. However, stimulant laxatives are exclusively designated for as-needed use. Probiotics, bulk-forming laxatives, prokinetics, and Kampo medicines, for which there is insufficient evidence, are considered alternative or complementary therapy. Providing the best clinical strategies for chronic constipation therapy in Japan, these clinical guidelines for chronic constipation 2023 should prove useful for its treatment worldwide. The Japan Gastroenterological Association published the first version of its clinical guidelines for chronic constipation 2023. Based on the latest evidence, these guidelines describe the definition, classification, diagnostic criteria, diagnostic testing methods, epidemiology, pathophysiology, and treatment of chronic constipation. They include flowcharts for both diagnosis and treatment of chronic constipation. In the treatment of chronic constipation, the first step involves differentiating between secondary forms, such as organic disease-associated constipation, systemic disease-associated constipation, and drug-induced constipation. The next step is to determine whether the chronic constipation stems from a motility disorder, a form of primary chronic constipation. For functional constipation and constipation-predominant irritable bowel syndrome, treatment should be initiated after evaluating symptoms like reduced bowel movement frequency type or defecation difficulty type. The first line of treatment includes the improvement of lifestyle habits and diet therapy. The first drugs to consider for oral treatment are osmotic laxatives. If these are ineffective, secretagogues and ileal bile acid transporter inhibitors are candidates. However, stimulant laxatives are exclusively designated for as-needed use. Probiotics, bulk-forming laxatives, prokinetics, and Kampo medicines, for which there is insufficient evidence, are considered alternative or complementary therapy. Providing the best clinical strategies for chronic constipation therapy in Japan, these clinical guidelines for chronic constipation 2023 should prove useful for its treatment worldwide.
Keywords: Chronic constipation; Constipation-predominant irritable bowel syndrome; Definition; Functional constipation; Guidelines.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Competing interests of guideline development committee members, guideline committee members, and guideline evaluation committee members were in the following. Any financial relationship with enterprises, businesses, or academic institutions in the subject matter or materials discussed in the manuscript are as follows: (1) those from which the authors, the spouse, partner, or immediate relatives of the authors have received individually any income, honoraria, or any other type of remuneration; Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company, EA Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., AstraZeneca K.K., Olympus Corporation, JIMRO Co., Ltd., AbbVie GK, KYORIN Pharmaceutical Co., Ltd., Viatris Inc., Astellas Pharma Inc., Gilead Sciences, Inc., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Tsumura & Co., Towa Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Kowa Pharmaceutical Co., Ltd., Taisho Pharmaceutical Holdings Co., Ltd., Biofermin Pharmaceutical Co., Ltd., Mylan EPD G.K., Daiichi Sankyo Inc., Zeria Pharmaceutical Co., Ltd., and (2) those from which the authors have received research grant; AbbVie GK, GlaxoSmithKline K.K., Janssen Pharmaceutical K.K., EA Pharma Co., Ltd., Fujifilm Corporation, Mochida Pharmaceutical Co., Ltd., ASKA Pharmaceutical Co., Ltd., Astellas Pharma Inc., Gilead Sciences, Inc., Biofermin Pharmaceutical Co., Ltd., Mylan EPD G.K., Tsumura & Co., Zespri International Limited, and (3) those from which the authors have received scholarship; Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Tobishima Kodomo Clinic, Takeda Pharmaceutical Company, Daiichi Sankyo Inc., AbbVie GK, KYORIN Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb K.K., EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., and (4) those from which the authors have received individually endowed chair; Ono Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Otsuka Pharmaceutical Factory, Inc., Fujifilm Medical Co., Ltd., Terumo Corporation, FANCL Corporation, Ohga Pharmacy, Abbott Japan LLC, and Muta Hospital.
Figures





References
-
- Li H, Xing X, Yao L, Li M, Xun Y, Yan P, et al. . Assessment of the quality and content of clinical practice guidelines on irritable bowel syndrome using the agree ii instrument. Digestion. 2020;101(4):355–65. - PubMed
-
- Yoshida M, Kinoshita Y, Watanabe M, Sugano K. Jsge clinical practice guidelines 2014: standards, methods, and process of developing the guidelines. J Gastroenterol. 2015;50(1):4–10. - PubMed
-
- Qaseem A, Kansagara D, Lin JS, Mustafa RA, Wilt TJ; Clinical Guidelines Committee of the American College of Physicians, et al. . The development of clinical guidelines and guidance statements by the clinical guidelines committee of the American college of physicians: update of methods. Ann Intern Med. 2019;170(12):863–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

