Dual therapeutic targeting of MYC and JUNB transcriptional programs for enhanced anti-myeloma activity
- PMID: 39160158
- PMCID: PMC11333473
- DOI: 10.1038/s41408-024-01117-4
Dual therapeutic targeting of MYC and JUNB transcriptional programs for enhanced anti-myeloma activity
Abstract
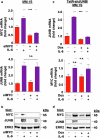
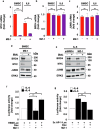
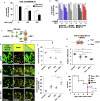
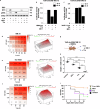
Deregulation of transcription factors (TFs) leading to uncontrolled proliferation of tumor cells within the microenvironment represents a hallmark of cancer. However, the biological and clinical impact of transcriptional interference, particularly in multiple myeloma (MM) cells, remains poorly understood. The present study shows for the first time that MYC and JUNB, two crucial TFs implicated in MM pathogenesis, orchestrate distinct transcriptional programs. Specifically, our data revealed that expression levels of MYC, JUNB, and their respective downstream targets do not correlate and that their global chromatin-binding patterns are not significantly overlapping. Mechanistically, MYC expression was not affected by JUNB knockdown, and conversely, JUNB expression and transcriptional activity were not affected by MYC knockdown. Moreover, suppression of MYC levels in MM cells via targeting the master regulator BRD4 by either siRNA-mediated knockdown or treatment with the novel proteolysis targeting chimera (PROTAC) MZ-1 overcame bone marrow (BM) stroma cell/IL-6-induced MYC- but not MEK-dependent JUNB-upregulation and transcriptional activity. Consequently, targeting of the two non-overlapping MYC- and JUNB-transcriptoms by MZ-1 in combination with genetic or pharmacological JUNB-targeting approaches synergistically enhanced MM cell death, both in 2D and our novel dynamic 3D models of the BM milieu as well as in murine xenografts. In summary, our data emphasize the opportunity to employ MYC and JUNB dual-targeting treatment strategies in MM as another exciting approach to further improve patient outcomes.
© 2024. The Author(s).
Conflict of interest statement
SV received speaker’s honoraria from Bristol Myers Squibb, MSD, and Pfizer, and consultancy fees from Roche, Eusa, MSD, and Merck; KP has received speaker’s honoraria from Celgene, Amgen Inc., and Janssen Pharmaceuticals, consultancy fees from Celgene, Takeda and Janssen Pharmaceuticals, and research support from Roche Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

