SIRPα engagement regulates ILC2 effector function and alleviates airway hyperreactivity via modulating energy metabolism
- PMID: 39160226
- PMCID: PMC11442993
- DOI: 10.1038/s41423-024-01208-z
SIRPα engagement regulates ILC2 effector function and alleviates airway hyperreactivity via modulating energy metabolism
Abstract
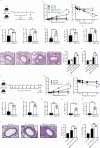
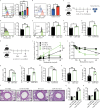
Group-2 innate lymphoid cells (ILC2) are part of a growing family of innate lymphocytes known for their crucial role in both the development and exacerbation of allergic asthma. The activation and function of ILC2s are regulated by various activating and inhibitory molecules, with their balance determining the severity of allergic responses. In this study, we aim to elucidate the critical role of the suppressor molecule signal regulatory protein alpha (SIRPα), which interacts with CD47, in controlling ILC2-mediated airway hyperreactivity (AHR). Our data indicate that activated ILC2s upregulate the expression of SIRPα, and the interaction between SIRPα and CD47 effectively suppresses both ILC2 proliferation and effector function. To evaluate the function of SIRPα in ILC2-mediated AHR, we combined multiple approaches including genetically modified mouse models and adoptive transfer experiments in murine models of allergen-induced AHR. Our findings suggest that the absence of SIRPα leads to the overactivation of ILC2s. Conversely, engagement of SIRPα with CD47 reduces ILC2 cytokine production and effectively regulates ILC2-dependent AHR. Furthermore, the SIRPα-CD47 axis modulates mitochondrial metabolism through the JAK/STAT and ERK/MAPK signaling pathways, thereby regulating NF-κB activity and the production of type 2 cytokines. Additionally, our studies have revealed that SIRPα is inducible and expressed on human ILC2s, and administration of human CD47-Fc effectively suppresses the effector function and cytokine production. Moreover, administering human CD47-Fc to humanized ILC2 mice effectively alleviates AHR and lung inflammation. These findings highlight the promising therapeutic potential of targeting the SIRPα-CD47 axis in the treatment of ILC2-dependent allergic asthma.
Keywords: AHR; Asthma; CD47; ILC2; SIRPa.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Backman H, Jansson SA, Stridsman C, Eriksson B, Hedman L, Eklund BM, et al. Severe asthma-A population study perspective. Clin Exp Allergy. 2019;49:819–28. - PubMed
-
- Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of Severe Asthma Worldwide: Data From the International Severe Asthma Registry. Chest. 2020;157:790–804. - PubMed
-
- Cao Y, Li Y, Wang X, Liu S, Zhang Y, Liu G, et al. Dopamine inhibits group 2 innate lymphoid cell-driven allergic lung inflammation by dampening mitochondrial activity. Immunity. 2023;56:320–35.e9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

