Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson's disease: a blinded randomized feasibility trial
- PMID: 39160351
- PMCID: PMC11826929
- DOI: 10.1038/s41591-024-03196-z
Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson's disease: a blinded randomized feasibility trial
Abstract
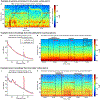
Deep brain stimulation (DBS) is a widely used therapy for Parkinson's disease (PD) but lacks dynamic responsiveness to changing clinical and neural states. Feedback control might improve therapeutic effectiveness, but the optimal control strategy and additional benefits of 'adaptive' neurostimulation are unclear. Here we present the results of a blinded randomized cross-over pilot trial aimed at determining the neural correlates of specific motor signs in individuals with PD and the feasibility of using these signals to drive adaptive DBS. Four male patients with PD were recruited from a population undergoing DBS implantation for motor fluctuations, with each patient receiving adaptive DBS and continuous DBS. We identified stimulation-entrained gamma oscillations in the subthalamic nucleus or motor cortex as optimal markers of high versus low dopaminergic states and their associated residual motor signs in all four patients. We then demonstrated improved motor symptoms and quality of life with adaptive compared to clinically optimized standard stimulation. The results of this pilot trial highlight the promise of personalized adaptive neurostimulation in PD based on data-driven selection of neural signals. Furthermore, these findings provide the foundation for further larger clinical trials to evaluate the efficacy of personalized adaptive neurostimulation in PD and other neurological disorders. ClinicalTrials.gov registration: NCT03582891 .
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures









Update of
-
Personalized chronic adaptive deep brain stimulation outperforms conventional stimulation in Parkinson's disease.medRxiv [Preprint]. 2023 Aug 8:2023.08.03.23293450. doi: 10.1101/2023.08.03.23293450. medRxiv. 2023. Update in: Nat Med. 2024 Nov;30(11):3345-3356. doi: 10.1038/s41591-024-03196-z. PMID: 37649907 Free PMC article. Updated. Preprint.
References
-
- Marceglia S. et al. Deep brain stimulation: is it time to change gears by closing the loop? J. Neural Eng 18, (2021). - PubMed
-
- Stanslaski S. et al. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc 20, 410–421 (2012). - PubMed
Methods-only References
-
- Oehrn CR, Cernera S, Hammer LH, Shcherbakova M, Yao J, Hahn A, Wang S, Ostrem JL, Little S, & Starr PA Chronic adaptive deep brain stimulation is superior to conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial [Source Data]. Data Archive for the Brain Initiative. 10.18120/cq9c-d057 (2024). - DOI - PMC - PubMed
-
- Oehrn CR, Cernera S, Hammer LH, Shcherbakova M, Yao J, Hahn A, Wang S, Ostrem JL, Little S, & Starr PA Chronic adaptive deep brain stimulation is superior to conventional stimulation in Parkinson's disease: a blinded randomized feasibility trial [Source Code]. Code Ocean. 10.24433/CO.5656158.v1 (2024). - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- R25NS070680/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- K23NS120037/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS131405-01/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R25 NS070680/NS/NINDS NIH HHS/United States
- U24 NS113637/NS/NINDS NIH HHS/United States
- R01 NS090913/NS/NINDS NIH HHS/United States
- UH3NS100544/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- F32 NS129627/NS/NINDS NIH HHS/United States
- R01 NS131405/NS/NINDS NIH HHS/United States
- UH3 NS100544/NS/NINDS NIH HHS/United States
- F32NS129627/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- K23 NS120037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

