Assessing the efficacy of tocotrienol-rich fraction vitamin E in obese children with non-alcoholic fatty liver disease: a single-blind, randomized clinical trial
- PMID: 39160468
- PMCID: PMC11334366
- DOI: 10.1186/s12887-024-04993-8
Assessing the efficacy of tocotrienol-rich fraction vitamin E in obese children with non-alcoholic fatty liver disease: a single-blind, randomized clinical trial
Abstract
Background: Childhood obesity is a growing concern, and non-alcoholic fatty liver disease (NAFLD) is a significant consequence. Currently, there are no approved drugs to treat NAFLD in children. However, a recent study explored the potential of vitamin E enriched with tocotrienol (TRF) as a powerful antioxidant for NAFLD. The aims of the present study were to investigate the effectiveness and safety of TRF in managing children with obesity and NAFLD.
Methods: A total of 29 patients aged 10 to 18 received a daily oral dose of 50 mg TRF for six months (January 2020 to February 2022), and all had fatty liver disease were detected by ultrasonography and abnormally high alanine transaminase levels (at least two-fold higher than the upper limits for their respective genders). Various parameters, including biochemical markers, FibroScan, LiverFASt, DNA damage, and cytokine expression, were monitored.
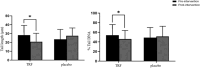
Results: APO-A1 and AST levels decreased significantly from 1.39 ± 0.3 to 1.22 ± 0.2 g/L (P = 0.002) and from 30 ± 12 to 22 ± 10 g/L (P = 0.038), respectively, in the TRF group post-intervention. Hepatic steatosis was significantly reduced in the placebo group from 309.38 ± 53.60 db/m to 277.62 ± 39.55 db/m (p = 0.048), but not in the TRF group. Comet assay analysis showed a significant reduction in the DNA damage parameters in the TRF group in the post-intervention period compared to the baseline, with tail length decreasing from 28.34 ± 10.9 to 21.69 ± 9.84; (p = 0.049) and with tail DNA (%) decreasing from 54.13 ± 22.1to 46.23 ± 17.9; (p = 0.043). Pro-inflammatory cytokine expression levels were significantly lower in the TRF group compared to baseline levels for IL-6 (2.10 6.3 to 0.7 1.0 pg/mL; p = 0.047 pg/mL) and TNF-1 (1.73 5.5 pg/mL to 0.7 0.5 pg/mL; p = 0.045).
Conclusion: The study provides evidence that TRF supplementation may offer a risk-free treatment option for children with obesity and NAFLD. The antioxidant and anti-inflammatory properties of TRF offer a promising adjuvant therapy for NAFLD treatment. In combination with lifestyle modifications such as exercise and calorie restriction, TRF could play an essential role in the prevention of NAFLD in the future. However, further studies are needed to explore the long-term effects of TRF supplementation on NAFLD in children.
Trial registration: The study has been registered with the International Clinical Trial Registry under reference number (NCT05905185) retrospective registration on (15/06/2023).
Keywords: Anti-inflammatory agent; Antioxidants; Non-alcoholic fatty liver disease; Oxidative stress; Tocotrienols.
© 2024. The Author(s).
Conflict of interest statement
The authors hereby declare no conflict of interest.
Figures

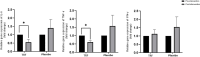
References
-
- Marcinkiewicz K, Horodnicka-Józwa A, Jackowski T, Strączek K, Biczysko-Mokosa A, Walczak M, Petriczko E. Nonalcoholic fatty liver disease in children with obesity–observations from one clinical centre in the Western Pomerania region. Front Endocrinol. 2022;13: 992264.10.3389/fendo.2022.992264 - DOI - PMC - PubMed
-
- Li J, Ren W. Diagnosis and therapeutic strategies for non-alcoholic fatty liver disease in children. Zhonghua gan Zang Bing za zhi= Zhonghua Ganzangbing Zazhi= Chinese. J Hepatol. 2020;28(3):208–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

