The tripartite motif-containing 24 is a multifunctional player in human cancer
- PMID: 39160596
- PMCID: PMC11334367
- DOI: 10.1186/s13578-024-01289-3
The tripartite motif-containing 24 is a multifunctional player in human cancer
Abstract
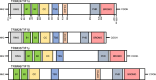
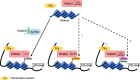
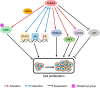
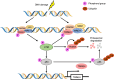
Tripartite motif-containing 24 (TRIM24), also known as transcriptional intermediary factor 1α (TIF1α), is the founding member of TIF1 family. Recent evidence indicates that aberrant expression of TRIM24, functions as an oncogene, is associated with poor prognosis across various cancer types. TRIM24 exhibits a multifaceted structure comprising an N-terminal TRIM region with a RING domain, B-box type 1 and type 2 domains, and a coiled-coil region, as well as a C-terminal plant-homeodomain (PHD)-bromodomain. The bromodomain serves as a 'reader' of epigenetic histone marks, regulating chromatin structure and gene expression by linking associated proteins to acetylated nucleosomal targets, thereby controlling transcription of genes. Notably, bromodomains have emerged as compelling targets for cancer therapeutic development. In addition, TRIM24 plays specialized roles as a signal transduction molecule, orchestrating various cellular signaling cascades in cancer cells. Herein, we review the recent advancements in understanding the functions of TRIM24, and demonstrate the research progress in utilizing TRIM24 as a target for cancer therapy.
Keywords: Cancer treatment; Cell proliferation; Epithelial–mesenchymal transition; TRIM24; Transcription regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

