Defining Vaginal Community Dynamics: daily microbiome transitions, the role of menstruation, bacteriophages, and bacterial genes
- PMID: 39160615
- PMCID: PMC11331738
- DOI: 10.1186/s40168-024-01870-5
Defining Vaginal Community Dynamics: daily microbiome transitions, the role of menstruation, bacteriophages, and bacterial genes
Abstract
Background: The composition of the vaginal microbiota during the menstrual cycle is dynamic, with some women remaining eu- or dysbiotic and others transitioning between these states. What defines these dynamics, and whether these differences are microbiome-intrinsic or mostly driven by the host is unknown. To address this, we characterized 49 healthy, young women by metagenomic sequencing of daily vaginal swabs during a menstrual cycle. We classified the dynamics of the vaginal microbiome and assessed the impact of host behavior as well as microbiome differences at the species, strain, gene, and phage levels.
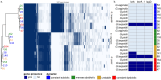
Results: Based on the daily shifts in community state types (CSTs) during a menstrual cycle, the vaginal microbiome was classified into four Vaginal Community Dynamics (VCDs) and reported in a classification tool, named VALODY: constant eubiotic, constant dysbiotic, menses-related, and unstable dysbiotic. The abundance of bacteria, phages, and bacterial gene content was compared between the four VCDs. Women with different VCDs showed significant differences in relative phage abundance and bacterial composition even when assigned to the same CST. Women with unstable VCDs had higher phage counts and were more likely dominated by L. iners. Their Gardnerella spp. strains were also more likely to harbor bacteriocin-coding genes.
Conclusions: The VCDs present a novel time series classification that highlights the complexity of varying degrees of vaginal dysbiosis. Knowing the differences in phage gene abundances and the genomic strains present allows a deeper understanding of the initiation and maintenance of permanent dysbiosis. Applying the VCDs to further characterize the different types of microbiome dynamics qualifies the investigation of disease and enables comparisons at individual and population levels. Based on our data, to be able to classify a dysbiotic sample into the accurate VCD, clinicians would need two to three mid-cycle samples and two samples during menses. In the future, it will be important to address whether transient VCDs pose a similar risk profile to persistent dysbiosis with similar clinical outcomes. This framework may aid interdisciplinary translational teams in deciphering the role of the vaginal microbiome in women's health and reproduction. Video Abstract.
Keywords: Daily variations; Dysbiosis; Menstrual cycle; Reproductive health; Vaginal microbiome.
© 2024. The Author(s).
Conflict of interest statement
The Centre for Translational Microbiome Research is partly funded by Ferring Pharmaceuticals (LWH, EF, JD, LE, IS-K). An unrestricted research grant from Ferring Pharmaceuticals enabled the clinical infrastructure and sampling (MCK, ZB and HSN). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures







References
-
- Tamarelle J, et al. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: a systematic review and meta-analysis. Clin Microbiol Infecti. 2019;25:35–47. Preprint at : 10.1016/j.cmi.2018.04.019. 10.1016/j.cmi.2018.04.019 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

