Nicotinamide N-methyltransferase inhibition mitigates obesity-related metabolic dysfunction
- PMID: 39161060
- PMCID: PMC11622326
- DOI: 10.1111/dom.15879
Nicotinamide N-methyltransferase inhibition mitigates obesity-related metabolic dysfunction
Abstract
Aim: To assess the effects of a small-molecule nicotinamide N-methyltransferase (NNMT) inhibitor, 5A1MQ, on body composition, metabolic variables, fatty liver pathologies, and circulating biomarkers in diet-induced obese (DIO) mice, and characterize its plasma pharmacokinetics (PK) and tissue distribution in vivo.
Materials and methods: DIO mice were administered vehicle or 5A1MQ once daily for 28 days. Longitudinal measures of body composition, blood glucose and plasma insulin levels, and terminal measures of liver histopathology and serum markers, were evaluated. Plasma and tissue PK were established in age- and strain-matched mice after intravenous, oral, and subcutaneous dosing of 5A1MQ.
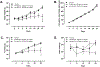
Results: 5A1MQ treatment dose-dependently limited body weight and fat mass gains, improved oral glucose tolerance and insulin sensitivity, and suppressed hyperinsulinaemia in DIO mice. Liver histology from 5A1MQ-treated DIO mice showed attenuated hepatic steatosis and macrophage infiltration, and correspondingly reduced liver weight, size, and triglyceride levels. 5A1MQ treatment normalized circulating levels of alanine transaminase, aspartate transaminase, and ketone bodies, supporting an overall improvement in liver and metabolic functions. The pharmacodynamic effects of 5A1MQ were further corroborated by its high systemic exposure and effective distribution to metabolically active tissues, including adipose, muscle and liver, following subcutaneous dosing of mice.
Conclusions: This work validates NNMT inhibition as a viable pharmacological approach to ameliorate metabolic imbalances and improve liver pathologies that develop with obesity.
Keywords: animal pharmacology; antiobesity drug; drug development; pharmacodynamics; pharmacokinetics; type 2 diabetes.
© 2024 John Wiley & Sons Ltd.
Conflict of interest statement
Competing Interests
SJW is the founder of Ridgeline Therapeutics, HN is a paid employee of Ridgeline Therapeutics, and JJB and DB are former employees of Ridgeline Therapeutics. HLS declares no competing interests.
Figures




References
-
- Cersosimo E, Triplitt C, Solis-Herrera C, Mandarino LJ, DeFronzo RA. Pathogenesis of Type 2 Diabetes Mellitus. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. South Dartmouth (MA)2000.
-
- DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37(6):667–687. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

