Carbon-phosphorus stapled Au(I) anticancer agents via bisphosphine induced reductive elimination
- PMID: 39161271
- PMCID: PMC11333934
- DOI: 10.1039/d4dt01929f
Carbon-phosphorus stapled Au(I) anticancer agents via bisphosphine induced reductive elimination
Abstract
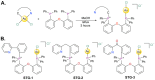
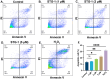
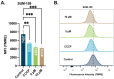
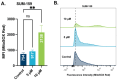
Towards the goal of generating new stabilized gold complexes as potent anticancer agents, we report here a novel class of Au(I) agents from Au(III)-mediated Caryl-P bond formation captured within the same complex by reacting a C^N cyclometalated Au(III) complex with bisphosphines. Cyclometalated Au(III) complexes of the type [Au(C^N)Cl2], where C^N represent different aryl pyridine framework reacted with bis(2-diphenylphosphino)phenyl ether in refluxing methanol to access an unsymmetrical gold complex featuring C-P coupling and Au(I)-phosphine. The complexes were characterized by 1H-NMR, 13C-NMR, and 31P-NMR and mass spectrometry. The structures of the complexes were characterized by X-ray crystallography and purity ascertained by HPLC and elemental analysis. The complexes demonstrate promising anticancer activity in a broad panel of cancer cell lines of different tumor origin. Mechanistically, the complexes induce apoptosis, generate mitochondrial ROS, depolarize mitochondrial membrane potential and modulate mitochondrial respiration in cancer cells. Overall, we developed a new structural class of Au(I) complexes with promising anticancer potential with potential utility in other applications.
Conflict of interest statement
The authors declare the following competing financial interest(s): S. G. A. has patents pending to the University of Kentucky Research Foundation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

