Multicomponent syntheses of pyrazoles via (3 + 2)-cyclocondensation and (3 + 2)-cycloaddition key steps
- PMID: 39161713
- PMCID: PMC11331544
- DOI: 10.3762/bjoc.20.178
Multicomponent syntheses of pyrazoles via (3 + 2)-cyclocondensation and (3 + 2)-cycloaddition key steps
Abstract
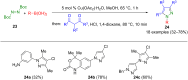
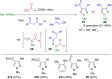
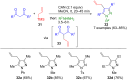
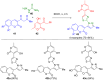
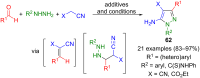
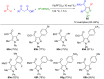
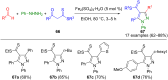
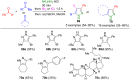
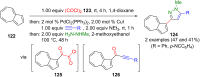
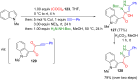
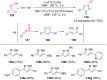
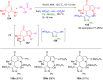
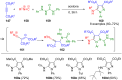
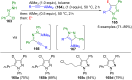
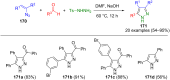
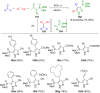
Pyrazoles are rarely found in nature but are traditionally used in the agrochemical and pharmaceutical industries, while other areas of use are also actively developing. However, they have also found numerous other applications. The search for new and efficient syntheses of these heterocycles is therefore highly relevant. The modular concept of multicomponent reactions (MCR) has paved a broad alley to heteroaromatics. The advantages over traditional methods are the broader scope and increased efficiency of these reactions. In particular, traditional multistep syntheses of pyrazoles have considerably been extended by MCR. Progress has been made in the cyclocondensation of 1,3-dielectrophiles that are generated in situ. Limitations in the regioselectivity of cyclocondensation with 1,3-dicarbonyls were overcome by the addition-cyclocondensation of α,β-unsaturated ketones. Embedding 1,3-dipolar cycloadditions into a one-pot process has additionally been developed for concise syntheses of pyrazoles. The MCR strategy also allows for concatenating classical condensation-based methodology with modern cross-coupling and radical chemistry, as well as providing versatile synthetic approaches to pyrazoles. This overview summarizes the most important MCR syntheses of pyrazoles based on ring-forming sequences in a flashlight fashion.
Keywords: cycloaddition; cyclocondensation; multicomponent reaction; one-pot reactions; pyrazole.
Copyright © 2024, Betcke et al.
Figures






































Similar articles
-
Multicomponent Hetero-[4 + 2] Cycloaddition/Allylboration Reaction: From Natural Product Synthesis to Drug Discovery.Acc Chem Res. 2016 Nov 15;49(11):2489-2500. doi: 10.1021/acs.accounts.6b00403. Epub 2016 Oct 18. Acc Chem Res. 2016. PMID: 27753496
-
Titanium-catalyzed multicomponent couplings: efficient one-pot syntheses of nitrogen heterocycles.Acc Chem Res. 2015 Nov 17;48(11):2822-33. doi: 10.1021/acs.accounts.5b00280. Epub 2015 Aug 21. Acc Chem Res. 2015. PMID: 26295382
-
Trifluoromethyl-β-dicarbonyls as Versatile Synthons in Synthesis of Heterocycles.Chemistry. 2024 Feb 21;30(11):e202303599. doi: 10.1002/chem.202303599. Epub 2024 Jan 4. Chemistry. 2024. PMID: 38055226 Review.
-
Multicomponent synthesis of chromophores - The one-pot approach to functional π-systems.Front Chem. 2023 Mar 17;11:1124209. doi: 10.3389/fchem.2023.1124209. eCollection 2023. Front Chem. 2023. PMID: 37007054 Free PMC article. Review.
-
Deconstructive Synthesis of Bridged and Fused Rings via Transition-Metal-Catalyzed "Cut-and-Sew" Reactions of Benzocyclobutenones and Cyclobutanones.Acc Chem Res. 2022 Aug 16;55(16):2341-2354. doi: 10.1021/acs.accounts.2c00400. Epub 2022 Jul 28. Acc Chem Res. 2022. PMID: 35901263 Free PMC article.
Cited by
-
Innovative Silica-Supported Acid Catalyst for Sustainable Synthesis of Bioactive Pyrazoles: Insights into Mechanisms and Applications.ACS Omega. 2025 May 8;10(19):19750-19763. doi: 10.1021/acsomega.5c00920. eCollection 2025 May 20. ACS Omega. 2025. PMID: 40415834 Free PMC article.
-
Synthesis of Perfluoroalkylated Pyrazoles from α-Perfluoroalkenylated Aldehydes.Molecules. 2024 Oct 25;29(21):5034. doi: 10.3390/molecules29215034. Molecules. 2024. PMID: 39519675 Free PMC article.
-
Unraveling aromaticity: the dual worlds of pyrazole, pyrazoline, and 3D carborane.Beilstein J Org Chem. 2025 Feb 21;21:412-420. doi: 10.3762/bjoc.21.29. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 39996167 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
