Coupled oscillations orchestrate selective information transmission in visual cortex
- PMID: 39161729
- PMCID: PMC11331424
- DOI: 10.1093/pnasnexus/pgae288
Coupled oscillations orchestrate selective information transmission in visual cortex
Abstract
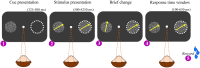
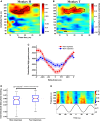
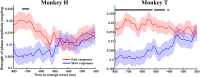
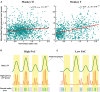
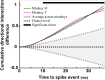
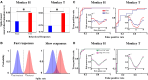
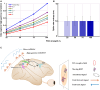
Performing visually guided behavior involves flexible routing of sensory information towards associative areas. We hypothesize that in visual cortical areas, this routing is shaped by a gating influence of the local neuronal population on the activity of the same population's single neurons. We analyzed beta frequencies (representing local population activity), high-gamma frequencies (representative of the activity of local clusters of neurons), and the firing of single neurons in the medial temporal (MT) area of behaving rhesus monkeys. Our results show an influence of beta activity on single neurons, predictive of behavioral performance. Similarly, the temporal dependence of high-gamma on beta predicts behavioral performance. These demonstrate a unidirectional influence of network-level neural dynamics on single-neuron activity, preferentially routing relevant information. This demonstration of a local top-down influence unveils a previously unexplored perspective onto a core feature of cortical information processing: the selective transmission of sensory information to downstream areas based on behavioral relevance.
Keywords: high-gamma oscillations; local field potentials; macaque visual area MT; neural oscillations; phase-amplitude coupling (PAC).
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures
References
LinkOut - more resources
Full Text Sources

