Advances and challenges in anti-cancer vaccines for multiple myeloma
- PMID: 39161773
- PMCID: PMC11331005
- DOI: 10.3389/fimmu.2024.1411352
Advances and challenges in anti-cancer vaccines for multiple myeloma
Abstract
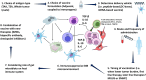
Multiple myeloma (MM) is a hematological cancer marked by plasma cell accumulation in the bone marrow. Despite treatment advancements, MM remains incurable in most patients. MM-associated immune dysregulation fosters disease progression, prompting research into immunotherapy to combat the disease. An area of immunotherapy investigation is the design of myeloma vaccine therapy to reverse tumor-associated immune suppression and elicit tumor-specific immune responses to effectively target MM cells. This article reviews vaccine immunotherapy for MM, categorizing findings by antigen type and delivery method. Antigens include idiotype (Id), tumor-associated (TAA), tumor-specific (TSA), and whole tumor lysate. Myeloma vaccination has so far shown limited clinical efficacy. However, further studies are essential to optimize various aspects, including antigen and patient selection, vaccine timing and sequencing, and rational combinations with emerging MM treatments.
Keywords: idiotype (Id); immunotherapy; multiple myeloma (MM); tumor-associated antigen (TAA); tumor-specific antigen (TSA); vaccine.
Copyright © 2024 Abdollahi, Norseth and Schjesvold.
Conflict of interest statement
HN: Honoraria: Janssen, BMS and Sanofi. FS: Honoraria: Amgen, BMS, Takeda, Abbvie, Janssen, Novartis, SkyliteDX, Oncopeptides, Sanofi, Schain, Pfizer, Daiki-Sankyo; Consultancy: GSK, Celgene, Takeda, Janssen, Oncopeptides, Sanofi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

