The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference
- PMID: 39161804
- PMCID: PMC11330755
- DOI: 10.2147/PPA.S470091
The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference
Erratum in
-
Erratum: The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference [Corrigendum].Patient Prefer Adherence. 2025 Mar 28;19:805-807. doi: 10.2147/PPA.S529186. eCollection 2025. Patient Prefer Adherence. 2025. PMID: 40171517 Free PMC article.
Abstract
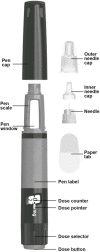
Introduction: Concizumab is a once-daily prophylactic treatment developed for patients with hemophilia A or B (HA/HB) with or without inhibitors. It is the first treatment for hemophilia patients to be delivered subcutaneously using a pre-filled, multi-dose pen-injector with a 4 mm, 32 G needle.
Aim: To investigate patient and caregiver handling and preference for the concizumab pen-injector compared with current injection systems used to treat hemophilia.
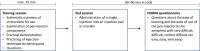
Methods: This preference and handling study was conducted in accordance with authority guidelines for approval of new devices and included adults and adolescents with HA/HB with or without inhibitors and caregivers currently administering factor replacement therapy or factor VIII mimetic (emicizumab) therapy. All participants underwent a training session, followed by a test session during which participants independently administered a single pen-injection into an injection pad or manikin. Time to train, time to prepare and inject, and number of complete independent injections handling the pen were assessed. Participants evaluated handling and preference via the Hemophilia Device Handling and Preference Assessment Questionnaire.
Results: 80 participants (44 adults, 21 adolescents, 15 caregivers) currently using factor replacement therapy (n=41, 51%) or emicizumab (n=39, 49%) participated. Average training time and time to complete an injection were 7 min 49s and 1 min 21s. In total, 98% of independent complete injections were achieved at first attempt. 98% (n=78; 95% confidence interval [CI] 91-100%) of participants assessed the pen-injector as either "easy" or "very easy" to use. 88% of participants preferred the pen-injector (n=70; 95% CI 78-94%) over their current injection system, and 9% (n=7) reported "no preference".
Conclusion: Participants found the concizumab pen-injector easy to learn and easy to use and preferred it over their current injection systems.
Keywords: concizumab; pen-injector; preference and handling study; questionnaire; subcutaneous.
Plain language summary
People with hemophilia lack clotting factors that help stop bleeding after an injury. If left untreated, it can lead to life-threatening bleeding episodes. Treatment such as replacement therapy with clotting factors needs to be administered at regular intervals to prevent bleeding. The administration of intravenous (inside the vein) medication can be challenging due to the required skills, injection pain and availability of a good vein to inject into. Factor VIII mimetic therapy now allows people to inject medication under the skin (subcutaneously). However, using vials and syringes still poses challenges with the time to prepare and inject medication. Pen-injectors have been developed to address these challenges and provide additional benefits, including increased dose accuracy, portability, reduced discomfort, and less administration time. Concizumab is a medication developed for once-daily subcutaneous injection for people with hemophilia A or hemophilia B, with or without inhibitors, using a pen-injector. The objective of this preference and handling study was to understand patient and caregiver pen-injector handling experiences, and device preferences compared to their existing devices. In this study, most participants found it easy to learn to use the pen-injector. 98% of participants reported that the pen-injector was easy or very easy to use compared with their current device. 88% of participants reported a preference for the concizumab pen-injector over their current device. This shows that the pen-injector can easily and effectively be used to inject concizumab, providing more control and independence in patients’ treatment while reducing the burden caused by intravenous modes of administration.
© 2024 Kahr Rasmussen et al.
Conflict of interest statement
NKR, BB, ASLC, JSN, GTB, and TS are employees of Novo Nordisk. EH and MG are employees of Research Collective, LLC, Tempe, Arizona, USA. Novo Nordisk is the sponsor of the trial. The study was conducted by an independent CRO. The authors report no other conflicts of interest in this work.
Figures




References
LinkOut - more resources
Full Text Sources

