Anthocyanins-rich cranberry extract attenuates DSS-induced IBD in an intestinal flora independent manner
- PMID: 39161885
- PMCID: PMC11332073
- DOI: 10.1016/j.crfs.2024.100815
Anthocyanins-rich cranberry extract attenuates DSS-induced IBD in an intestinal flora independent manner
Abstract
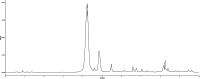
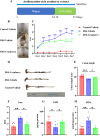
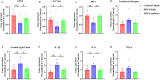
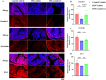
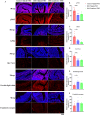
Cranberry is abundantly rich in anthocyanins, a type of flavonoid with potent antioxidant properties and the resistance against certain diseases. In this study, anthocyanin-rich cranberry extract was extracted, purified, and its components were analyzed. 92.18 % of anthocyanins was obtained and the total content of anthocyanins was 302.62 mg/g after AB-8 resin purification. Quantification analysis showed that the extract mainly contained cyanidin-3-galactoside, procyanidin B2 and procyanidin B4. Then we explored its effects on dextran sulfate sodium (DSS)-induced inflammatory bowel disease (IBD) in mice. The supplementation of cranberry extract resulted in an alleviation of IBD symptoms, evidenced by improvements in the disease activity index (DAI), restoration of colon length and colonic morphology. Cranberry extract reversed the elevated iron and malondialdehyde (MDA) levels and restored glutathione (GSH) levels in IBD mice. Further analysis revealed that cranberry modulated ferroptosis-associated genes and reduced expression of pro-inflammatory cytokines. Although cranberry influenced the intestinal flora balance by reducing Proteobacteria and Escherichia-Shigella, and increasing Lactobacillus, as well as enhancing SCFAs content, these effects were not entirely dependent on intestinal flora modulation, as indicated by antibiotic intervention and fecal microbiota transplantation (FMT) experiments. In conclusion, our findings suggest that the beneficial impact of cranberry extract on IBD may primarily involve the regulation of colonic ferroptosis, independent of significant alterations in intestinal flora.
Keywords: Anthocyanins; Cranberry; Fecal microbiota transplantation (FMT); Ferroptosis; Inflammatory bowel disease (IBD).
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Abdelhalim K.A. Short-chain fatty acids (SCFAs) from gastrointestinal disorders, metabolism, epigenetics, central nervous system to cancer - a mini-review. Chem. Biol. Interact. 2024;388 - PubMed
-
- Alemany-Cosme E., Saez-Gonzalez E., Moret I., Mateos B., Iborra M., Nos P., Sandoval J., Beltran B. Oxidative stress in the pathogenesis of crohn's disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants. 2021;10 - PMC - PubMed
-
- Barnes J.S., Nguyen H.P., Shen S., Schug K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A. 2009;1216:4728–4735. - PubMed
-
- Breugelmans T., Van Spaendonk H., De Man J.G., De Schepper H.U., Jauregui-Amezaga A., Macken E., Linden S.K., Pintelon I., Timmermans J.P., De Winter B.Y., Smet A. In-depth study of transmembrane mucins in association with intestinal barrier dysfunction during the course of T cell transfer and DSS-induced colitis. J Crohns Colitis. 2020;14:974–994. - PubMed
LinkOut - more resources
Full Text Sources

