Trametinib Potentiates Anti-PD-1 Efficacy in Tumors Established from Chemotherapy-Primed Pancreatic Cancer Cells
- PMID: 39162011
- PMCID: PMC11614707
- DOI: 10.1158/1535-7163.MCT-23-0833
Trametinib Potentiates Anti-PD-1 Efficacy in Tumors Established from Chemotherapy-Primed Pancreatic Cancer Cells
Abstract
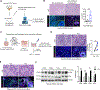
Despite advances in immune checkpoint inhibitors, chemotherapy remains the standard therapy for patients with pancreatic ductal adenocarcinoma (PDAC). As the combinations of chemotherapy, including the FOLFIRINOX [5-fluorouracil, F; irinotecan, I; and oxaliplatin, O (FIO)] regimen, and immune checkpoint inhibitors have failed to demonstrate clinical benefit in patients with metastatic PDAC tumors, there is increasing interest in identifying therapeutic approaches to potentiate ICI efficacy in patients with PDAC. In this study, we report that neoadjuvant FOLFIRINOX-treated human PDAC tumors exhibit increased MEK/ERK activation. We also show elevated MEK/ERK signaling in ex vivo PDAC slice cultures and cell lines treated with a combination of FIO. In addition, we find that the KPC-FIO cells, established from repeated treatment of mouse PDAC cell lines with six to eight cycles of FIO, display enhanced ERK phosphorylation and demonstrate increased sensitivity to MEK inhibition in vitro and in vivo. Significantly, the KPC-FIO cells develop tumors with a proinflammatory immune profile similar to human PDAC tumors after neoadjuvant FOLFIRINOX treatment. Furthermore, we found that the MEK inhibitor trametinib enables additional infiltration of highly functional CD8+ T cells into the KPC-FIO tumors and potentiates the efficacy of anti-PD-1 antibody in syngeneic mouse models. Our findings provide a rationale for combining trametinib and anti-PD-1 antibodies in patients with PDAC after neoadjuvant or short-term FOLFIRINOX treatment to achieve effective antitumor responses.
©2024 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures



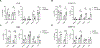

References
-
- Ghaneh P, Palmer D, Cicconi S, Jackson R, Halloran CM, Rawcliffe C, Sripadam R, Mukherjee S, Soonawalla Z, Wadsley J, et al. (2023). Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol 8, 157–168. 10.1016/S2468-1253(22)00348-X. - DOI - PubMed
-
- Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, et al. (2016). Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 151, e161137. 10.1001/jamasurg.2016.1137. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- I21BX005693/U.S. Department of Veterans Affairs (VA)
- I01BX005595/U.S. Department of Veterans Affairs (VA)
- I01 BX005595/BX/BLRD VA/United States
- IRG-21-144-270/American Cancer Society (ACS)
- I21 BX005693/BX/BLRD VA/United States
- R21 CA255291/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- T32CA070085/National Cancer Institute (NCI)
- R01 CA265997/CA/NCI NIH HHS/United States
- T32 CA070085/CA/NCI NIH HHS/United States
- Lurie Cancer Center Diversity Scholars Award/Robert H. Lurie Comprehensive Cancer Center (LCC)
- Robert and Lora Lurie Endowed Professorship/Northwestern University (NU)
- R01CA265997/National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

