Tensing Flipper: Photosensitized Manipulation of Membrane Tension, Lipid Phase Separation, and Raft Protein Sorting in Biological Membranes
- PMID: 39162019
- PMCID: PMC11363133
- DOI: 10.1021/jacs.4c08580
Tensing Flipper: Photosensitized Manipulation of Membrane Tension, Lipid Phase Separation, and Raft Protein Sorting in Biological Membranes
Abstract
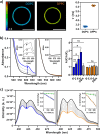
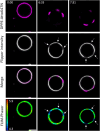
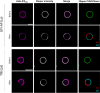
The lateral organization of proteins and lipids in the plasma membrane is fundamental to regulating a wide range of cellular processes. Compartmentalized ordered membrane domains enriched with specific lipids, often termed lipid rafts, have been shown to modulate the physicochemical and mechanical properties of membranes and to drive protein sorting. Novel methods and tools enabling the visualization, characterization, and/or manipulation of membrane compartmentalization are crucial to link the properties of the membrane with cell functions. Flipper, a commercially available fluorescent membrane tension probe, has become a reference tool for quantitative membrane tension studies in living cells. Here, we report on a so far unidentified property of Flipper, namely, its ability to photosensitize singlet oxygen (1O2) under blue light when embedded into lipid membranes. This in turn results in the production of lipid hydroperoxides that increase membrane tension and trigger phase separation. In biological membranes, the photoinduced segregated domains retain the sorting ability of intact phase-separated membranes, directing raft and nonraft proteins into ordered and disordered regions, respectively, in contrast to radical-based photo-oxidation reactions that disrupt raft protein partitioning. The dual tension reporting and photosensitizing abilities of Flipper enable simultaneous visualization and manipulation of the mechanical properties and lateral organization of membranes, providing a powerful tool to optically control lipid raft formation and to explore the interplay between membrane biophysics and cell function.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes.Biochim Biophys Acta. 2012 Jul;1818(7):1777-84. doi: 10.1016/j.bbamem.2012.03.007. Biochim Biophys Acta. 2012. PMID: 22450237
-
Determination of lipid raft partitioning of fluorescently-tagged probes in living cells by Fluorescence Correlation Spectroscopy (FCS).J Vis Exp. 2012 Apr 6;(62):e3513. doi: 10.3791/3513. J Vis Exp. 2012. PMID: 22508446 Free PMC article.
-
Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies.Biochim Biophys Acta. 2005 Dec 30;1746(3):193-202. doi: 10.1016/j.bbamcr.2005.09.003. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16271405 Review.
-
Lipid Peroxidation Drives Liquid-Liquid Phase Separation and Disrupts Raft Protein Partitioning in Biological Membranes.J Am Chem Soc. 2024 Jan 17;146(2):1374-1387. doi: 10.1021/jacs.3c10132. Epub 2024 Jan 3. J Am Chem Soc. 2024. PMID: 38171000 Free PMC article.
-
Lipid-protein interplay and lateral organization in biomembranes.Chem Phys Lipids. 2015 Jul;189:48-55. doi: 10.1016/j.chemphyslip.2015.05.008. Epub 2015 May 30. Chem Phys Lipids. 2015. PMID: 26036778 Review.
Cited by
-
Targeting lipid scrambling potentiates ferroptosis and triggers tumor immune rejection.Sci Adv. 2025 Aug 15;11(33):eadx6587. doi: 10.1126/sciadv.adx6587. Epub 2025 Aug 15. Sci Adv. 2025. PMID: 40815641 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

