Trapped in Endosome PEGylated Ultra-Small Iron Oxide Nanoparticles Enable Extraordinarily High MR Imaging Contrast for Hepatocellular Carcinomas
- PMID: 39162181
- PMCID: PMC11497028
- DOI: 10.1002/advs.202401351
Trapped in Endosome PEGylated Ultra-Small Iron Oxide Nanoparticles Enable Extraordinarily High MR Imaging Contrast for Hepatocellular Carcinomas
Abstract
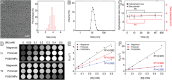
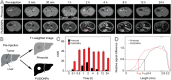
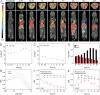
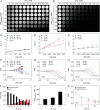
The early diagnosis of hepatocellular carcinomas (HCCs) remains challenging in the clinic. Primovist-enhanced magnetic resonance imaging (MRI) aids HCC diagnosis but loses sensitivity for tumors <2 cm. Therefore, developing advanced MRI contrast agents is imperative for improving the diagnostic accuracy of HCCs in very-early-stage. To address this challenge, PEGylated ultra-small iron oxide nanoparticles (PUSIONPs) are synthesized and employed as liver-specific T1 MRI contrast agents. Intravenous delivery produces simultaneous hyperintense HCC and hypointense hepatic parenchyma signals on T1 imaging, creating an extraordinarily high tumor-to-liver contrast. Systematic studies uncover PUSIONP distribution in hepatic parenchyma, HCC lesions at the organ, tissue, cellular, and subcellular levels, revealing endosomal confinement of PUSIONP without aggregation. By mimicking such situations, the dependency of relaxometric properties on local PUSIONP concentration is investigated, emphasizing the key role of different endosomal concentrations in liver and tumor cells for high tumor-to-liver contrast and clear tumor boundaries. These findings offer exceptional imaging capabilities for early HCC diagnosis, potentially benefiting real HCC patients.
Keywords: contrast agent; early diagnosis; hepatocellular carcinoma; iron oxide nanoparticle.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., CA Cancer J. Clin. 2022, 72, 7; - PubMed
- b) Lu J., Sun J., Li F., Wang J., Liu J., Kim D., Fan C., Hyeon T., Ling D., J. Am. Chem. Soc. 2018, 140, 10071; - PubMed
- c) Lee Y. L., Lee J. M., Lee J. S., Lee H. Y., Park B. H., Kim Y. H., Han J. K., Choi B. I., Radiology 2015, 275, 97; - PubMed
- d) Forner A., Llovet J. M., Bruix J., Lancet 2012, 379, 1245. - PubMed
-
- a) Galle P. R., Forner A., Llovet J. M., Mazzaferro V., Piscaglia F., Raoul J. L., Schirmacher P., Vilgrain V., J. Hepatol. 2018, 69, 182; - PubMed
- b) Marrero J. A., Kulik L. M., Sirlin C. B., Zhu A. X., Finn R. S., Abecassis M. M., Roberts L. R., Heimbach J. K., Hepatology 2018, 68, 723; - PubMed
- c) Reig M., Forner A., Rimola J., Ferrer‐Fabrega J., Burrel M., Garcia‐Criado A., Kelley R. K., Galle P. R., Mazzaferro V., Salem R., Sangro B., Singal A. G., Vogel A., Fuster J., Ayuso C., Bruix J., J. Hepatol. 2022, 76, 681; - PMC - PubMed
- d) Nur Syafiqah Abu Bakar F. S. S., Mahmud M. H., Healthscope 2019, 1, 143;
- e) Jia Z., Song L., Zang F., Song J., Zhang W., Yan C., Xie J., Ma Z., Ma M., Teng G., Gu N., Zhang Y., Theranostics 2016, 6, 1780. - PMC - PubMed
-
- El‐Serag H. B., Marrero J. A., Rudolph L., Reddy K. R., Gastroenterology 2008, 134, 1752. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
