Calcium-binding protein TgpCaBP regulates calcium storage of the zoonotic parasite Toxoplasma gondii
- PMID: 39162521
- PMCID: PMC11448132
- DOI: 10.1128/spectrum.00661-24
Calcium-binding protein TgpCaBP regulates calcium storage of the zoonotic parasite Toxoplasma gondii
Abstract
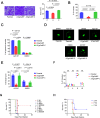
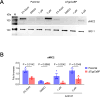
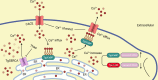
Toxoplasma gondii, the causative parasite of toxoplasmosis, is an apicomplexan parasite that infects warm-blooded mammals. The ability of the calcium-binding proteins (CBPs) to transport large amounts of Ca2+ appears to be critical for the biological activity of T. gondii. However, the functions of some members of the CBP family have not yet been deciphered. Here, we characterized a putative CBP of T. gondii, TgpCaBP (TGME49_229480), which is composed of four EF-hand motifs with Ca2+-binding capability. TgpCaBP was localized in the cytosol and ER of T. gondii, and parasites lacking the TgpCaBP gene exhibited diminished abilities in cell invasion, intracellular growth, egress, and motility. These phenomena were due to the abnormalities in intracellular Ca2+ efflux and ER Ca2+ storage, and the reduction in motility was associated with a decrease in the discharge of secretory proteins. Therefore, we propose that TgpCaBP is a Ca2+ transporter and signaling molecule involved in Ca2+ regulation and parasitization in the hosts.IMPORTANCECa2+ signaling is essential in the development of T. gondii. In this study, we identified a calcium-binding protein in T. gondii, named TgpCaBP, which actively regulates intracellular Ca2+ levels in the parasite. Deletion of the gene coding for TgpCaBP caused serious deficits in the parasite's ability to maintain a stable intracellular calcium environment, which also impaired the secretory protein discharged from the parasite, and its capacity of gliding motility, cell invasion, intracellular growth, and egress from host cells. In summary, we have identified a novel calcium-binding protein, TgpCaBP, in the zoonotic parasite T. gondii, which is a potential therapeutic target for toxoplasmosis.
Keywords: Ca2+ signaling; TgpCaBP; Toxoplasma gondii; calcium binding; parasite biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Orthologs of Plasmodium ICM1 are dispensable for Ca2+ mobilization in Toxoplasma gondii.Microbiol Spectr. 2024 Oct 3;12(10):e0122924. doi: 10.1128/spectrum.01229-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162502 Free PMC article.
-
The TgAMPK-TgPFKII axis essentially regulates protein lactylation in the zoonotic parasite Toxoplasma gondii.Microbiol Spectr. 2025 Mar 4;13(3):e0204424. doi: 10.1128/spectrum.02044-24. Epub 2025 Feb 7. Microbiol Spectr. 2025. PMID: 39918324 Free PMC article.
-
A Member of the Ferlin Calcium Sensor Family Is Essential for Toxoplasma gondii Rhoptry Secretion.mBio. 2018 Oct 2;9(5):e01510-18. doi: 10.1128/mBio.01510-18. mBio. 2018. PMID: 30279285 Free PMC article.
-
Calcium signaling and the lytic cycle of the Apicomplexan parasite Toxoplasma gondii.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1846-1856. doi: 10.1016/j.bbamcr.2018.08.004. Epub 2018 Aug 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30992126 Free PMC article. Review.
-
Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells.Parasitol Int. 1999 Mar;48(1):1-10. doi: 10.1016/s1383-5769(98)00042-7. Parasitol Int. 1999. PMID: 11269320 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

