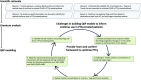
Challenges with sirolimus experimental data to inform QSP model of post-transplantation cyclophosphamide regimens
- PMID: 39162578
- PMCID: PMC11334299
- DOI: 10.1111/cts.70014
Challenges with sirolimus experimental data to inform QSP model of post-transplantation cyclophosphamide regimens
Abstract
Dose optimization of sirolimus may further improve outcomes in allogeneic hematopoietic cell transplant (HCT) patients receiving post-transplantation cyclophosphamide (PTCy) to prevent graft-versus-host disease (GVHD). Sirolimus exposure-response association studies in HCT patients (i.e., the association of trough concentration with clinical outcomes) have been conflicting. Sirolimus has important effects on T-cells, including conventional (Tcons) and regulatory T-cells (Tregs), both of which have been implicated in the mechanisms by which PTCy prevents GVHD, but there is an absence of validated biomarkers of sirolimus effects on these cell subsets. Considering the paucity of existing biomarkers and the complexities of the immune system, we conducted a literature review to inform a quantitative systems pharmacology (QSP) model of GVHD. The published literature presented multiple challenges. The sirolimus pharmacokinetic models insufficiently describe sirolimus distribution to relevant physiological compartments. Despite multiple publications describing sirolimus effects on Tcons and Tregs in preclinical and human ex vivo models, consistent parameters relating sirolimus concentrations to circulating Tcons and Tregs could not be found. Each aspect presents a challenge in building a QSP model of sirolimus and its temporal effects on T-cell subsets and GVHD prevention. To optimize GVHD prevention regimens, phase I studies and systematic studies of immunosuppressant concentration-effect association are needed for QSP modeling.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests in this work.
Figures