Quantitative 68Ga-PSMA-11 PET and Clinical Outcomes in Metastatic Castration-resistant Prostate Cancer Following 177Lu-PSMA-617 (VISION Trial)
- PMID: 39162634
- PMCID: PMC11366674
- DOI: 10.1148/radiol.233460
Quantitative 68Ga-PSMA-11 PET and Clinical Outcomes in Metastatic Castration-resistant Prostate Cancer Following 177Lu-PSMA-617 (VISION Trial)
Abstract
Background Lutetium 177 [177Lu]Lu-PSMA-617 (177Lu-PSMA-617) is a prostate-specific membrane antigen (PSMA)-targeted radioligand therapy for metastatic castration-resistant prostate cancer (mCRPC). Quantitative PSMA PET/CT analysis could provide information on 177Lu-PSMA-617 treatment benefits. Purpose To explore the association between quantitative baseline gallium 68 [68Ga]Ga-PSMA-11 (68Ga-PSMA-11) PET/CT parameters and treatment response and outcomes in the VISION trial. Materials and Methods This was an exploratory secondary analysis of the VISION trial. Eligible participants were randomized (June 2018 to October 2019) in a 2:1 ratio to 177Lu-PSMA-617 therapy (7.4 GBq every 6 weeks for up to six cycles) plus standard of care (SOC) or to SOC only. Baseline 68Ga-PSMA-11 PET parameters, including the mean and maximum standardized uptake value (SUVmean and SUVmax), PSMA-positive tumor volume, and tumor load, were extracted from five anatomic regions and the whole body. Associations of quantitative PET parameters with radiographic progression-free survival (rPFS), overall survival (OS), objective response rate, and prostate-specific antigen response were investigated using univariable and multivariable analyses (with treatment as the only other covariate). Outcomes were assessed in subgroups based on SUVmean quartiles. Results Quantitative PET parameters were well balanced between study arms for the 826 participants included. The median whole-body tumor SUVmean was 7.6 (IQR, 5.8-9.9). Whole-body tumor SUVmean was the best predictor of 177Lu-PSMA-617 efficacy, with a hazard ratio (HR) range of 0.86-1.43 for all outcomes (all P < .001). A 1-unit whole-body tumor SUVmean increase was associated with a 12% and 10% decrease in risk of an rPFS event and death, respectively. 177Lu-PSMA-617 plus SOC prolonged rPFS and OS in all SUVmean quartiles versus SOC only, with no identifiable optimum among participants receiving 177Lu-PSMA-617. Higher baseline PSMA-positive tumor volume and tumor load were associated with worse rPFS (HR range, 1.44-1.53 [P < .05] and 1.02-1.03 [P < .001], respectively) and OS (HR range, 1.36-2.12 [P < .006] and 1.04 [P < .001], respectively). Conclusion Baseline 68Ga-PSMA-11 PET/CT whole-body tumor SUVmean was the best predictor of 177Lu-PSMA-617 efficacy in participants in the VISION trial. Improvements in rPFS and OS with 177Lu-PSMA-617 plus SOC were greater among participants with higher whole-body tumor SUVmean, with evidence for benefit at all SUVmean levels. ClinicalTrials.gov identifier: NCT03511664 Published under a CC BY 4.0 license. Supplemental material is available for this article.
Conflict of interest statement
Figures

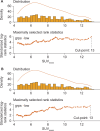
![Segmentation of anatomic regions and distribution of quantitative
68Ga-PSMA-11 PET parameters. (A) Whole-body anterior coronal
prostate-specific membrane antigen (PSMA) PET maximum intensity projection
images in a 63-year-old White male participant with tumors in the liver,
bone, and lymph node (LN) who had an initial prostate-specific antigen level
of 181.9 ng/mL and Eastern Cooperative Oncology Group performance score of
0/1 show all PSMA-positive (PSMA+) disease as a single whole-body volume in
red (left) and segmented according to anatomic region (right; bone lesions
in blue, liver lesions in green, lymph node lesions in red). (B) Box plots
show the distribution of quantitative 68Ga-PSMA-11 PET parameters for the
study sample (n = 826) included in this analysis. Data are reported as
medians and IQRs from the full analysis sets for bone (n = 761), liver (n =
109), lymph node (n = 559), soft tissue (n = 334), and whole body (n = 826)
and stratified according to treatment (T; 177Lu-PSMA-617 plus standard of
care [SOC]), control (C; SOC only), and overall (O; all participants).
SUVmax = maximum standardized uptake value, SUVmean = mean standardized
uptake value.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/01dc/11366674/f6eccf7d9f81/radiol.233460.fig2.gif)

![Chart shows median radiographic progression-free survival (rPFS) and
overall survival (OS) according to whole-body tumor mean standardized uptake
value (SUVmean) quartile, indicating statistically significant differences
in the three upper quartiles for both rPFS and OS but not the lowest
quartile. SUVmean quartiles were derived from the SUVmean of both study arms
combined (177Lu-PSMA-617 plus standard of care [SOC] and SOC only). The
statistical significance of the hazard ratios (HRs) for each quartile is
indicated by 95% CIs that exclude unity. NE = not evaluable, PSMA =
prostate-specific membrane antigen.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/01dc/11366674/6aa48effc519/radiol.233460.fig4.gif)

References
-
- Horoszewicz JS , Kawinski E , Murphy GP . Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients . Anticancer Res 1987. ; 7 ( 5B ): 927 – 935 . - PubMed
-
- Israeli RS , Powell CT , Fair WR , Heston WD . Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen . Cancer Res 1993. ; 53 ( 2 ): 227 – 230 . - PubMed
-
- Minner S , Wittmer C , Graefen M , et al . High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer . Prostate 2011. ; 71 ( 3 ): 281 – 288 . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

