Multi-institutional prospective observational study of radiotherapy for metastatic bone tumor
- PMID: 39162649
- PMCID: PMC11420848
- DOI: 10.1093/jrr/rrae060
Multi-institutional prospective observational study of radiotherapy for metastatic bone tumor
Abstract
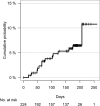
Purpose of this study is to evaluate patient characteristics, treatments and outcomes in bone metastasis radiotherapy practice. Patients for whom radiotherapy for bone metastasis was planned at 26 institutions in Japan between December 2020 and March 2021 were consecutively registered in this prospective, observational study. Study measures included patient characteristics, pain relief, skeletal-related events (SREs), overall survival and incidence of radiation-related adverse events. Pain was evaluated using a numerical rating scale (NRS) from 0 to 10. Irradiated dose was analyzed by the biologically effective dose (BED) assuming α/β = 10. Overall, 232 patients were registered; 224 patients and 302 lesions were fully analyzed. Eastern Cooperative Oncology Group Performance Status was 0/1/2/3/4 in 23%/38%/22%/13%/4%; 59% of patients had spinal metastases and 84% had painful lesions (NRS ≥ 2). BED was <20 Gy (in 27%), 20-30 Gy (24%), 30-40 Gy (36%) and ≥ 40 Gy (13%); 9% of patients were treated by stereotactic body radiotherapy. Grade 3 adverse events occurred in 4% and no grade 4-5 toxicity was reported. Pain relief was achieved in 52% at 2 months. BED is not related to pain relief. The cumulative incidence of SREs was 6.5% (95% confidence interval (CI) 3.1-9.9) at 6 months; no factors were significantly associated with SREs. With spinal lesions, 18% of patients were not ambulatory at baseline and 50% of evaluable patients in this group could walk at 2 months. The 6-month overall survival rate was 70.2% (95% CI 64.2-76.9%). In conclusion, we report real-world details of radiotherapy in bone metastasis.
Keywords: SRE; bone metastasis; pain relief; prospective study; radiotherapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Japanese Radiation Research Society and Japanese Society for Radiation Oncology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Hideyuki Harada received honoraria from Astrazeneca, Accuray, Chugai pharmaceutical, Takeda pharmaceutical, MSD, Eizai pharmaceutical, Phizer, Brainlab, Hitachi, Novartis, Guerbet Japan, GE Healthcare, Nihon Medi-Physics and Taiho pharmaceutical outside this work. Naoto Shikama reports research grants from Chiyoda Technol, Boston Scientific, and Elekta during the conduct of this study. Yukinori Okada received lecture fee from PDRadiopharma Inc outside this work.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

