Loss of periostin function impairs ligament fibroblast activity and facilitates ROS-mediated cellular senescence
- PMID: 39162681
- PMCID: PMC11346584
- DOI: 10.1096/fj.202302615RR
Loss of periostin function impairs ligament fibroblast activity and facilitates ROS-mediated cellular senescence
Abstract
Anterior cruciate ligament (ACL) injuries pose a significant challenge due to their limited healing potential, often resulting in premature arthritis. The factors and mechanisms contributing to this inadequate healing process remain elusive. During the acute phase of injury, ACL tissues express elevated periostin levels that decline over time. The functional significance of periostin in ligament biology remains understudied. In this study, we investigated the functional and mechanistic implications of periostin deficiency in ACL biology, utilizing ligament fibroblasts derived from patients and a murine model of ACL rupture. Our investigations unveiled that periostin knockdown compromised fibroblast growth characteristics, hindered the egress of progenitor cells from explants, and arrested cell-cycle progression, resulting in the accumulation of cells in the G0/G1 phase and moderate apoptosis. Concurrently, a significant reduction in the expression of cell-cycle and matrix-related genes was observed. Moreover, periostin deficiency triggered apoptosis through STAT3Y705/p38MAPK signaling and induced cellular senescence through increased production of reactive oxygen species (ROS). Mechanistically, inhibition of ROS production mitigated cell senescence in these cells. Notably, in vivo data revealed that ACL in Postn-/- mice exhibited a higher tearing frequency than wild-type mice under equivalent loading conditions. Furthermore, injured ACL with silenced periostin expression, achieved through nanoparticle-siRNA complex delivery, displayed an elevated propensity for apoptosis and senescence compared to intact ACL in C57BL/6 mice. Together, our findings underscore the pivotal role of periostin in ACL health, injury, and potential for healing.
Keywords: ACL; ROS; STAT3; cell‐cycle; p16INK4A; p38MAPK.
© 2024 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest regarding the contents of this article. All authors have read the journal’s policy on disclosure of potential conflicts of interest.
Figures








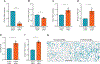


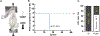

References
-
- Mall NA, Chalmers PN, Moric M, Tanaka MJ, Cole BJ, Bach BR Jr., and Paletta GA Jr. (2014) Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med 42, 2363–2370 - PubMed
-
- Lohmander LS, Englund PM, Dahl LL, and Roos EM (2007) The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35, 1756–1769 - PubMed
-
- Houck DA, Kraeutler MJ, Belk JW, Goode JA, Mulcahey MK, and Bravman JT (2019) Primary Arthroscopic Repair of the Anterior Cruciate Ligament: A Systematic Review of Clinical Outcomes. Arthroscopy 35, 3318–3327 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

