Fecal Immunochemical Test to Detect Colorectal Neoplasia in Lynch Syndrome: A Prospective Multicenter Study
- PMID: 39162771
- PMCID: PMC11864054
- DOI: 10.14309/ajg.0000000000003043
Fecal Immunochemical Test to Detect Colorectal Neoplasia in Lynch Syndrome: A Prospective Multicenter Study
Abstract
Introduction: Colonoscopy surveillance for Lynch syndrome is burdensome and postcolonoscopy colorectal cancers (CRCs) still occur. The noninvasive fecal immunochemical test (FIT) might guide optimal colonoscopy intervals.
Methods: Prospective, multicenter observational study in which individuals with Lynch syndrome performed a quantitative FIT before high-quality surveillance colonoscopy. Diagnostic performance of FIT at various thresholds ≤20 μg Hb/g feces was assessed for relevant neoplasia, including advanced neoplasia (CRC, advanced adenomas [AAs] and advanced serrated lesions [ASLs]) and non-advanced adenomas (NAAs).
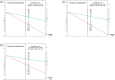
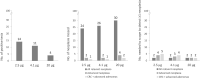
Results: Of the 217 included individuals (59% female, median age 51 years), 4 had CRC, 5 AA, 4 ASL, and 57 NAA as most relevant neoplasia. The lowest FIT positivity threshold (2.5 μg Hb/g feces, 14% positivity rate) maximized detection: 4/4 CRCs, 4/5 AA, 1/4 ASL, and 9/57 NAA were detected, resulting in a sensitivity and negative predictive value of, respectively, 89% and 99% for CRC plus AA, 69% and 97% for advanced neoplasia, and 26% and 72% for all relevant neoplasia (91% specificity for all groups). At equal sensitivity and negative predictive value, specificity for advanced neoplasia optimized to 94% at threshold 4.1 μg/g. Per 100 FITs at threshold 4.1 μg/g, 11 individuals would test positive and thus proceed to colonoscopy, 2 individuals with advanced neoplasia would be missed and 3 individuals would need colonoscopy to detect 1 advanced neoplasia.
Discussion: FIT at thresholds ≤4.1 μg Hb/g feces may be a promising strategy to postpone colonoscopy in approximately 9 of 10 individuals with Lynch syndrome. Large validation studies that also provide gene variant-specific outcomes should be prioritized.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures



References
-
- van Leerdam ME, Roos VH, van Hooft JE, et al. . Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019;51(11):1082–93. - PubMed
-
- Vasen H, Hes F, de Jong M. Dutch guideline for diagnostics and prevention of hereditary and familial tumours. 2017. (https://www.stoet.nl/wp-content/uploads/2017/04/STOET-Richtlijnenboekje-...). Accessed September 1, 2023.
-
- Denters MJ, Schreuder M, Depla AC, et al. . Patients' perception of colonoscopy: Patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol 2013;25(8):964–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

