Nivolumab plus chemotherapy in patients with HER2-negative, previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: 3-year follow-up of the ATTRACTION-4 randomized, double-blind, placebo-controlled, phase 3 trial
- PMID: 39162872
- PMCID: PMC11513732
- DOI: 10.1007/s10120-024-01535-0
Nivolumab plus chemotherapy in patients with HER2-negative, previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: 3-year follow-up of the ATTRACTION-4 randomized, double-blind, placebo-controlled, phase 3 trial
Abstract
Background: Nivolumab + chemotherapy is now a standard of care for HER2-negative, previously untreated, unresectable or recurrent gastric/gastroesophageal junction cancer (advanced gastric cancer), but long-term follow-up data of clinical trials are limited.
Methods: ATTRACTON-4 was a phase 3, double-blind, placebo-controlled trial in Japan, South Korea, and Taiwan. Patients were randomized to either nivolumab or placebo, both combined with the physician's choice of SOX (oral S-1 [tegafur-gimeracil-oteracil potassium] + oxaliplatin) or CAPOX (capecitabine + oxaliplatin). We report the primary endpoints-centrally assessed progression-free survival (PFS) and overall survival (OS)-and landmark analyses of OS among patients alive using 3-year follow-up data.
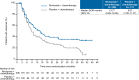
Results: At the cutoff date (May 10, 2021), 17/359 patients in the nivolumab + chemotherapy group and 6/358 in the placebo + chemotherapy group were continuing study treatment. PFS (centrally assessed) was longer in the nivolumab + chemotherapy group (median 10.94 vs. 8.48 months; hazard ratio [HR] 0.67, 95% confidence interval [CI] 0.55-0.82). Although OS did not differ between the two groups (median 17.45 vs. 17.15 months; HR 0.89, 95% CI 0.75-1.05), the landmark analysis of OS, calculating HRs at each landmark time point (every month), was getting numerically better in the nivolumab + chemotherapy group over time. Approximately 80% of patients who achieved complete response in the nivolumab + chemotherapy group were alive at 3 years. No new safety signals or major late-onset select treatment-related adverse events were observed for nivolumab + chemotherapy.
Conclusion: This 3-year follow-up of ATTRACTION-4 confirmed the long-term clinical benefit and manageable safety of nivolumab + chemotherapy in patients with previously untreated advanced gastric cancer.
Trial registration: NCT02746796.
Keywords: Chemotherapy; Gastric cancer; Nivolumab; Oxaliplatin; Randomized controlled trial.
© 2024. The Author(s).
Conflict of interest statement
Narikazu Boku disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; and honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, and Taiho Pharma. Takeshi Omori disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Kohei Shitara disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; research funding (to the institution) from Astellas Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharma, Chugai, MSD, Amgen, Eisai, PRA Health Sciences, Syneos Health; consulting fees as an advisor from Bristol-Myers Squibb, Takeda, Ono Pharmaceutical, Novartis, Daiichi Sankyo, Amgen, Boehringer Ingelheim, MSD, Astellas, Guardant Health Japan, Janssen, AstraZeneca, Zymeworks, ALX Oncology, and Bayer; and honoraria from Bristol-Myers Squibb, Ono Pharmaceutical, Janssen, Eli Lilly, Astellas, and AstraZeneca. Shinichi Sakuramoto disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Kensei Yamaguchi disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; grants from Taiho Pharma; and honoraria from Daiichi Sankyo, Chugai Pharmaceutical, Bristol-Myers Squibb, Eli Lilly, Taiho Pharma, Takeda, and Merck Biopharm. Ken Kato disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; consulting fees from Ono Pharmaceutical, Bristol-Myers Squibb, BeiGene/Novartis, AstraZeneca, Roche, Bayer, Merck & Co, Merck Bio, and Janssen; honoraria from Ono Pharmaceutical and Bristol-Myers Squibb; and payments for expert testimony from Ono Pharmaceutical and Bristol-Myers Squibb. Shigenori Kadowaki disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; research grants from Bristol-Myers Squibb, Ono Pharmaceutical, Bayer, Daiichi Sankyo, MSD, Chugai, Janssen, Nobelpharma, and Eli Lilly; and honoraria from Bristol-Myers Squibb, Ono Pharmaceutical, Bayer, Taiho Pharma, Eisai, Daiichi Sankyo, MSD, Chugai, and Otsuka Pharmaceutical. Kunihiro Tsuji disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Min-Hee Ryu disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; research grants from AstraZeneca; consulting fees from Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Eli Lilly, Taiho Pharma, Novartis, Daiichi Sankyo, AstraZeneca, Astellas, and Daehwa; and payments or honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Eli Lilly, Taiho Pharma, Novartis, and Daehwa. Do-Youn Oh disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; research grants from AstraZeneca, Novartis, Array, Eli Lilly, Servier, BeiGene, MSD, and Handok; and participation on data safety monitoring boards or advisory boards for AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharma, ASLAN, Halozyme, Zymeworks, Bristol-Myers Squibb, Celgene, BeiGene, Basilea, Turning Point, Yuhan, Arcus Biosciences, IQVIA, and MSD. Sang Cheul Oh disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Sun Young Rha disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; grants (to the institution) from AstraZeneca, Ono Pharmaceutical, Eisai, Ipsen, MSD, Merck KGaA, Pfizer, BeiGene, Astellas Pharma, AMGEN, ALX Oncology, Zymeworks, Macrogenics, Seagen, Bold Therapeutics, MedPacto, ABLBIO, Daiichi Sankyo, Taiho Pharmaceutical, Leap therapeutics, and Arcus Biosciences for conducting clinical trials; consulting fees from LG Biochem and Indivumed; personal fees/honoraria from MSD, Eli Lilly, Daiichi Sankyo, Eisai, and Ipsen; and participation on data safety monitoring boards or advisory boards for Amgen and Toray. Keun-Wook Lee disclosed institutional research funding (to the institution) from Ono Pharmaceutical for conducting clinical trials; research funding (to the institution) for conducting clinical trials from AstraZeneca, MSD, Merck KGaA, Roche, BeiGene, Leap therapeutics, ALX Oncology, Zymeworks, Astellas, Macrogenics, Amgen, Seagen, Bolt Therapeutics, Trishula Therapeutics, MedPacto, Green Cross Corp, Y-BIOLOGICS, Daiichi Sankyo, Taiho Phama, InventisBio, Elevar Therapeutics, Metafines, Idience, Genome & Company, and Exelixis; honoraria for lectures from Ono Pharmaceutical, Boryung, Daiichi Sankyo, Astellas, and Sanofi-Aventis; and participation on data safety monitoring boards or advisory boards for ALX Oncology and Metafines. Ik-Joo Chung disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Sun Jin Sym disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Li-Tzong Chen disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; grants (to the institution) from MSD; consulting fees from AstraZeneca and MSD; honoraria from AstraZeneca, MSD, Ono Pharmaceutical, and Bristol-Myers Squibb; participation on data safety monitoring boards or advisory boards for AstraZeneca and MSD; and receipt of study drugs (to the institute) from Ono Pharmaceutical, Bristol-Myers Squibb, and Boehringer Ingelheim. Jen-Shi Chen disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Li-Yuan Bai disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial. Takashi Nakada is an employee of Ono Pharmaceutical. Shunsuke Hagihara is an employee of Ono Pharmaceutical. Reina Makino is an employee of Ono Pharmaceutical. Eiji Nishiyama is an employee of Ono Pharmaceutical. Yoon-Koo Kang disclosed research funding (to the institution) from Ono Pharmaceutical for this clinical trial; medical writing support from Astellas Pharma; and consulting fees from Amgen, Novartis, Roche, Daehwa, Zymeworks, Blueprint, Surface Oncology, ALX Oncology, Macrogenics, Bristol-Myers Squibb, Merck, and Liscure.
Figures




References
-
- Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35. 10.1016/s1470-2045(18)30791-5. - DOI - PubMed
-
- Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol. 2016;34(5):443–51. 10.1200/jco.2015.62.6598. - DOI - PubMed
-
- Ryu MH, Baba E, Lee KH, Park YI, Boku N, Hyodo I, et al. Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol. 2015;26(10):2097–101. 10.1093/annonc/mdv316. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

