Sacubitril-Valsartan in Patients Requiring Hemodialysis
- PMID: 39163041
- PMCID: PMC11337068
- DOI: 10.1001/jamanetworkopen.2024.29237
Sacubitril-Valsartan in Patients Requiring Hemodialysis
Abstract
Importance: Randomized clinical trials have shown that sacubitril-valsartan reduces the risks of mortality and hospitalization in patients with heart failure with reduced ejection fraction (HFrEF), but patients with kidney failure requiring dialysis were excluded.
Objective: To investigate the comparative effectiveness of sacubitril-valsartan vs angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEIs or ARBs) in patients with HFrEF requiring hemodialysis.
Design, setting, and participants: This retrospective, 1:1 propensity score-matched comparative effectiveness study included patients who were 18 years or older with HFrEF, enrolled in Medicare Parts A, B, and D, and survived at least 90 days receiving in-center hemodialysis from July 8, 2015, to December 31, 2020. Patients were excluded for less than 180 days of continuous Medicare Parts A, B, and D primary payer coverage or prior dispensing of sacubitril-valsartan. Data analysis was conducted from September 23, 2023, to June 25, 2024.
Exposures: New use of sacubitril-valsartan vs new or continued use of ACEIs or ARBs.
Main outcomes and measures: The associations between initiation of sacubitril-valsartan therapy and all-cause mortality, cardiovascular mortality, all-cause hospitalization, and HF hospitalization were assessed using Cox proportional hazards regression models in a propensity score-matched sample.
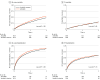
Results: Participants included 1:1 matched pairs of 1434 sacubitril-valsartan users and 1434 ACEI or ARB users (mean [SD] age, 64 [13] years). Of the 2868 matched participants, 996 (65%) were male; 987 (34%) were Black or African American and 1677 (58%) were White; and median dialysis vintage was 3.8 (IQR, 1.8-6.3) years. The median follow-up was 0.9 (IQR, 0.4-1.7) years. Sacubitril-valsartan (vs ACEI or ARB) therapy was associated with a reduction in all-cause mortality (hazard ratio [HR], 0.82 [95% CI, 0.73-0.92]) and all-cause hospitalization (HR, 0.86 [95% CI, 0.79-0.93]) but not cardiovascular mortality (HR, 1.01 [95% CI, 0.86-1.19]) or HF hospitalization (HR, 0.91 [95% CI, 0.82-1.02]). There was a decrease in hyperkalemia (HR, 0.71 [95% CI, 0.62-0.81]) and no difference in hypotension (HR, 0.99 [95% CI, 0.83-1.19]). Only 195 participants (14%) ever received the maximum combination dose of sacubitril (97 mg twice daily) and valsartan (103 mg twice daily).
Conclusions and relevance: In this comparative effectiveness study of patients with HFrEF requiring hemodialysis, sacubitril-valsartan therapy was associated with beneficial effects in all-cause mortality and all-cause hospitalization.
Conflict of interest statement
Figures

References
-
- U.S. Renal Data System . 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2021.
-
- Heidenreich PA, Bozkurt B, Aguilar D, et al. . 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. doi:10.1161/CIR.0000000000001063 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

