Rh(I)-Catalyzed Regio- and Enantioselective Ring Opening of Vinyl Cyclopropanes
- PMID: 39163089
- PMCID: PMC11378301
- DOI: 10.1021/jacs.4c09490
Rh(I)-Catalyzed Regio- and Enantioselective Ring Opening of Vinyl Cyclopropanes
Abstract
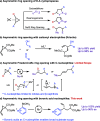
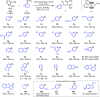
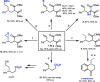
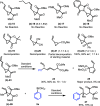
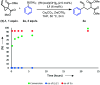
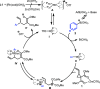
We describe a Rh(I) catalyzed asymmetric ring opening of racemic vinyl cyclopropanes using aryl boronic acids as C-nucleophiles. When ferrocene-based chiral bisphosphines are used as ligands, the products are obtained with regioselectivities typically 99:1 r.r. and ee's generally between 88 and 96%. A wide range of aryl boronic acids can be used, and the products can be converted into a variety of targets. Preliminary mechanistic studies indicate that Zn(OTf)2 plays a significant role in the reaction by promoting rhodium-ligand complex formation and accelerating the reaction. We expect this method and these mechanistic insights to be useful in the development of new asymmetric methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- de Meijere A. Bonding Properties of Cyclopropane and Their Chemical Consequences. Angew. Chem., Int. Ed. 1979, 18, 809–826. 10.1002/anie.197908093. - DOI
LinkOut - more resources
Full Text Sources

