Chemotherapy Drives Tertiary Lymphoid Structures That Correlate with ICI-Responsive TCF1+CD8+ T Cells in Metastatic Ovarian Cancer
- PMID: 39163092
- PMCID: PMC11701433
- DOI: 10.1158/1078-0432.CCR-24-1594
Chemotherapy Drives Tertiary Lymphoid Structures That Correlate with ICI-Responsive TCF1+CD8+ T Cells in Metastatic Ovarian Cancer
Abstract
Purpose: Patients with high-grade serous ovarian carcinoma (HGSOC) are virtually insensitive to immune checkpoint inhibitors (ICI) employed as standalone therapeutics, at least in part reflecting microenvironmental immunosuppression. Thus, conventional chemotherapeutics and targeted anticancer agents that not only mediate cytotoxic effects but also promote the recruitment of immune effector cells to the HGSOC microenvironment stand out as promising combinatorial partners for ICIs in this oncological indication.
Experimental design: We harnessed a variety of transcriptomic, spatial, and functional assays to characterize the differential impact of neoadjuvant paclitaxel-carboplatin on the immunological configuration of paired primary and metastatic HGSOC biopsies as compared to neoadjuvant chemotherapy (NACT)-naïve HGSOC samples from five independent patient cohorts.
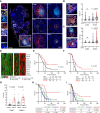
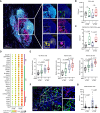
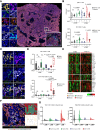
Results: We found NACT-driven endoplasmic reticulum stress and calreticulin exposure in metastatic HGSOC lesions culminates with the establishment of a dense immune infiltrate including follicular T cells (TFH cells), a prerequisite for mature tertiary lymphoid structure (TLS) formation. In this context, TLS maturation was associated with an increased intratumoral density of ICI-sensitive TCF1+PD1+ CD8+ T cells over their ICI-insensitive TIM-3+PD1+ counterparts. Consistent with this notion, chemotherapy coupled with a PD1-targeting ICI provided a significant survival benefit over either therapeutic approach in syngeneic models of HGSOC bearing high (but not low) tumor mutational burden.
Conclusions: Altogether, our findings suggest that NACT promotes TLS formation and maturation in HGSOC lesions, de facto preserving an intratumoral ICI-sensitive T-cell phenotype. These observations emphasize the role of rational design, especially relative to the administration schedule, for clinical trials testing chemotherapy plus ICIs in patients with HGSOC. See related commentary by Bravo Melgar and Laoui, p. 10.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Ryska reports personal fees from Amgen, AstraZeneca, BMS, Roche, MSD, Pfizer, Eli Lilly, MerckSerono, and Bayer and personal fees and non-financial support from Gilead and Sanofi outside the submitted work. M. Kovar reports grants from Sotio and Ministry of Education, Youth and Sports of the Czech Repblic and EU (Next Generation EU) during the conduct of the study as well as grants from Sotio outside the submitted work; in addition, M. Kovar has a patent for Methods and materials for targeted expansion of immune effector cells, International Application Number PCT/US2020/039857, pending. I.A. McNeish reports personal fees from GSK, AstraZeneca, Clovis Oncology, Pharma&, OncoC4, Roche, and Theolytics outside the submitted work. A. Coosemans reports grants from Novocure and Oncoinvent AS and personal fees from Molecular Partners, Sotio a.s., and Epics Therapeutics SA outside the submitted work; in addition, A. Coosemans has a patent for WO2021130356 (PCT/EP2020/087851: Disease Detection in Liquid Biopsies) pending. S. Orsulic reports grants from National Cancer Institute and Department of Veterans Administration during the conduct of the study. L. Galluzzi reports grants from Lytix, Promontory, and Onxeo and personal fees from AstraZeneca, AbbVie, OmniSEQ, The Longevity Labs, Inzen, Luke Heller TECPR2 Foundation, Sotio, Ricerchiamo, Noxopharm, Imvax, Boehringer Ingelheim, EduCom, and AbbVie outside the submitted work. No potential conflicts of interest were disclosed by the other authors.
Figures



References
-
- Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol 2019;30:672–705. - PubMed
-
- González-Martín A, Harter P, Leary A, Lorusso D, Miller RE, Pothuri B, et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:833–48. - PubMed
-
- Konstantinopoulos PA, Matulonis UA. Clinical and translational advances in ovarian cancer therapy. Nat Cancer 2023;4:1239–57. - PubMed
-
- Olalekan S, Xie B, Back R, Eckart H, Basu A. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep 2021;35:109165. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

