The phototrophic purple non-sulfur bacteria Rhodomicrobium spp. are novel chassis for bioplastic production
- PMID: 39163151
- PMCID: PMC11334908
- DOI: 10.1111/1751-7915.14552
The phototrophic purple non-sulfur bacteria Rhodomicrobium spp. are novel chassis for bioplastic production
Abstract
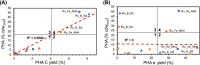
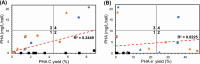
Petroleum-based plastics levy significant environmental and economic costs that can be alleviated with sustainably sourced, biodegradable, and bio-based polymers such as polyhydroxyalkanoates (PHAs). However, industrial-scale production of PHAs faces barriers stemming from insufficient product yields and high costs. To address these challenges, we must look beyond the current suite of microbes for PHA production and investigate non-model organisms with versatile metabolisms. In that vein, we assessed PHA production by the photosynthetic purple non-sulfur bacteria (PNSB) Rhodomicrobium vannielii and Rhodomicrobium udaipurense. We show that both species accumulate PHA across photo-heterotrophic, photo-hydrogenotrophic, photo-ferrotrophic, and photo-electrotrophic growth conditions, with either ammonium chloride (NH4Cl) or dinitrogen gas (N2) as nitrogen sources. Our data indicate that nitrogen source plays a significant role in dictating PHA synthesis, with N2 fixation promoting PHA production during photoheterotrophy and photoelectrotrophy but inhibiting production during photohydrogenotrophy and photoferrotrophy. We observed the highest PHA titres (up to 44.08 mg/L, or 43.61% cell dry weight) when cells were grown photoheterotrophically on sodium butyrate with N2, while production was at its lowest during photoelectrotrophy (as low as 0.04 mg/L, or 0.16% cell dry weight). We also find that photohydrogenotrophically grown cells supplemented with NH4Cl exhibit the highest electron yields - up to 58.89% - while photoheterotrophy demonstrated the lowest (0.27%-1.39%). Finally, we highlight superior electron conversion and PHA production compared to a related PNSB, Rhodopseudomonas palustris TIE-1. This study illustrates the value of studying non-model organisms like Rhodomicrobium for sustainable PHA production and indicates future directions for exploring PNSB metabolisms.
© 2024 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Update of
-
The phototrophic bacteria Rhodomicrobium spp. are novel chassis for bioplastic production.bioRxiv [Preprint]. 2023 May 17:2023.05.17.541187. doi: 10.1101/2023.05.17.541187. bioRxiv. 2023. Update in: Microb Biotechnol. 2024 Aug;17(8):e14552. doi: 10.1111/1751-7915.14552. PMID: 37292726 Free PMC article. Updated. Preprint.
References
-
- Bai, Y. , Zhou, L. , Irfan, M. , Liang, T.‐T. , Cheng, L. , Liu, Y.‐F. et al. (2020) Bioelectrochemical methane production from CO2 by Methanosarcina barkeri via direct and H2‐mediated indirect electron transfer. Energy, 210, 118445. Available from: 10.1016/j.energy.2020.118445 - DOI
Publication types
MeSH terms
Substances
Grants and funding
- DESC0014613/U.S. Department of Energy
- SEEDING PROJECTS FOR ENABLING EXCELLENCE & DISTINCTION (SPEED) - Washington University in St. Louis
- Collaboration Initiation Grant - Washington University in St. Louis
- Howard A. Schneiderman Fellowship
- W911NF-18-1-0037/U.S. Department of Defense
- R01 GM141344/GM/NIGMS NIH HHS/United States
- Gordon and Betty Moore Foundation
- 2124088/National Science Foundation
- FA9550-21-1-0211/Defense Established Program to Stimulate Competitive Research
- Office of the Vice-Chancellor of Research Grant - Washington University in St. Louis
- LLNL-JRNL-812309/U.S. Department of Energy
- 2117198/National Science Foundation
- 2300081/National Science Foundation
- 201563111/David and Lucile Packard Foundation
- 2021822/National Science Foundation
- International Center for Energy, Environment and Sustainability, Washington University in St. Louis
- NIHR01GM141344/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

