Computational detection of antigen-specific B cell receptors following immunization
- PMID: 39163333
- PMCID: PMC11363332
- DOI: 10.1073/pnas.2401058121
Computational detection of antigen-specific B cell receptors following immunization
Abstract
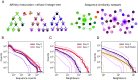
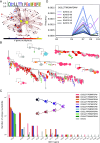
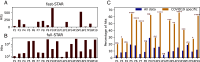
B cell receptors (BCRs) play a crucial role in recognizing and fighting foreign antigens. High-throughput sequencing enables in-depth sampling of the BCRs repertoire after immunization. However, only a minor fraction of BCRs actively participate in any given infection. To what extent can we accurately identify antigen-specific sequences directly from BCRs repertoires? We present a computational method grounded on sequence similarity, aimed at identifying statistically significant responsive BCRs. This method leverages well-known characteristics of affinity maturation and expected diversity. We validate its effectiveness using longitudinally sampled human immune repertoire data following influenza vaccination and SARS-CoV-2 infections. We show that different lineages converge to the same responding Complementarity Determining Region 3, demonstrating convergent selection within an individual. The outcomes of this method hold promise for application in vaccine development, personalized medicine, and antibody-derived therapeutics.
Keywords: COVID-19; adaptive immune system; antigen specificity; influenza vaccine; repertoire sequencing.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

