Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Breast Cancer During a 20-Year Follow-Up: A Population-Based Multicenter Retrospective Study
- PMID: 39163561
- PMCID: PMC11708989
- DOI: 10.1200/JCO.24.00171
Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Breast Cancer During a 20-Year Follow-Up: A Population-Based Multicenter Retrospective Study
Abstract
Purpose: Medication-related osteonecrosis of the jaw (MRONJ) is one of the most important toxicities of antiresorptive therapy, which is standard practice for patients with breast cancer and bone metastases. However, the population-based incidence of MRONJ is not well established. We therefore performed a retrospective multicenter study to assess the incidence for a whole Austrian federal state (Tyrol).
Materials and methods: This retrospective multicenter study was conducted between 2000 and 2020 at all nine breast centers across Tyrol, Austria. Using the cancer registry, the total Tyrolean population was screened for all patients with breast cancer. All patients with breast cancer and bone metastases receiving antiresorptive therapy were finally included in the study.
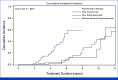
Results: From 8,860 patients initially screened, 639 individuals were eligible and included in our study. Patients received antiresorptive therapy once per month without de-escalation of therapy. MRONJ was diagnosed in 56 (8.8%, 95% CI, 6.6 to 11.0) patients. The incidence of MRONJ was 11.6% (95% CI, 8.0 to 15.3) in individuals treated with denosumab only, 2.8% (95% CI, 0.7 to 4.8) in those treated with bisphosphonates only, and 16.3% (95% CI, 8.8 to 23.9) in the group receiving bisphosphonates followed by denosumab. Individuals developed MRONJ significantly earlier when treated with denosumab. Time to MRONJ after treatment initiation was 4.6 years for individuals treated with denosumab only, 5.1 years for individuals treated with bisphosphonates only, and 8.4 years for individuals treated with both consecutively.
Conclusion: MRONJ incidence in breast cancer patients with bone metastases was found to be considerably higher, especially for patients receiving denosumab, when compared with available data in the literature. Additionally, patients treated with denosumab developed MRONJ significantly earlier.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- de Cassia Tornier S, Macedo FJ, Sassi LM, et al. : Quality of life in cancer patients with or without medication-related osteonecrosis of the jaw. Support Care Cancer 29:6713-6719, 2021 - PubMed
-
- Yao S, Ding X, Rong G, et al. : Association between malignant diseases and medication-related osteonecrosis of the jaw (MRONJ): A systematic review and meta-analysis. J Craniofac Surg 34:669-673, 2023 - PubMed
-
- Ng TL, Tu MM, Ibrahim MFK, et al. : Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support Care Cancer 29:925-943, 2021 - PubMed
-
- Henry D, Vadhan-Raj S, Hirsh V, et al. : Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: An analysis of data from patients with solid tumors. Support Care Cancer 22:679-687, 2014 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

