Outcome of patients with large B-cell lymphoma treated with tafasitamab plus lenalidomide either before or after CAR T-cell therapy
- PMID: 39163620
- PMCID: PMC11568786
- DOI: 10.1182/bloodadvances.2024013726
Outcome of patients with large B-cell lymphoma treated with tafasitamab plus lenalidomide either before or after CAR T-cell therapy
Abstract
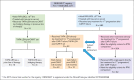
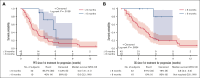
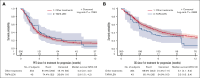
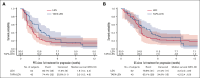
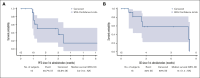
Tafasitamab plus lenalidomide (TAFA-LEN) treatment relevance pre- or post-anti-CD19 chimeric antigen receptor (CAR) T-cell therapy is debated. We analyzed patients with large B-cell lymphoma in the DESCAR-T registry treated with axi[1]cel or tisa-cel in ≥3rd line and TAFA-LEN before (n = 15, "TL-pre-CAR-T" set) or directly after (n = 52, "TL-post-CAR-T" set) CAR T-cell therapy. We compared TAFA-LEN v. other treatments using inverse probability weighting in the TL-post-CAR[1]T set. In the TL-post-CAR-T set, the median progression-free survival (mPFS), overall survival (mOS), and duration of response (mDOR) since the first treatment for progression (mPFS2/mOS2/mDOR2) were 3, 4.7, and 8.1 months, respectively. The best overall response rate (bORR) and best complete response rate (bCRR) after TAFA-LEN were 13.5% and 7.7%, respectively. Outcomes were better for patients who relapsed >6 months after CAR T-cell therapy (mPFS2: 5.6 vs 2 months, P = .0138; mOS2: not reached vs 3.8 months, P = .0034). The bORR and bCRR between TAFA-LEN and other treatments were 20.6% vs 24.9% and 11.6% vs 15.6%, respectively. Outcomes were similar between TAFA-LEN and other treatments (mPFS2: 2.9 vs 2.4 months, P = .91; mOS2: 3.3 vs 5.5 months, P = .06). In an exploratory analysis of the TL-pre-CAR-T set, the median TAFA-LEN treatment duration before CAR-T was 3.7 months with no patient becoming CD19 negative. The bORR, bCRR, 6- month PFS, and OS rates after CAR T-cell infusion were 45.5%, 36.4%, 20.1%, and 58.2%, respectively. Neither TAFA-LEN nor comparative salvage treatment improved outcomes for patients relapsing after CAR T-cell therapy.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: V.C. has received honoraria from Incyte, AbbVie, AstraZeneca, Bristol Myers Squibb, Ideogen, Janssen, Kyowa Kirin, Kite/Gilead, Lilly, Novartis, Octapharma, Pfizer, Pierre Fabre, Sanofi, and Takeda. P.S. has received honoraria from Chugai, BMS, Novartis, Janssen, Kite/Gilead, AbbVie, and Roche. J.P. has received honoraria from Incyte. L.D.L.R. has received honoraria from Novartis and Kite/Gilead. The remaining authors declare no competing financial interests.
Figures

References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Two-year follow-up of transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (liso-cel) in relapsed or refractory (R/R) large B-cell lymphomas (LBCL) Blood. 2021;138(suppl 1):2840–2843.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

