Vaginal Lactobacillus fatty acid response mechanisms reveal a metabolite-targeted strategy for bacterial vaginosis treatment
- PMID: 39163861
- PMCID: PMC11429459
- DOI: 10.1016/j.cell.2024.07.029
Vaginal Lactobacillus fatty acid response mechanisms reveal a metabolite-targeted strategy for bacterial vaginosis treatment
Abstract
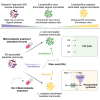
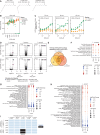
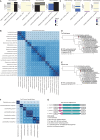
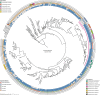
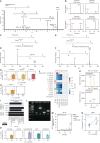
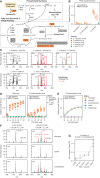
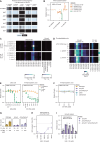
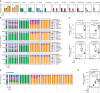
Bacterial vaginosis (BV), a common syndrome characterized by Lactobacillus-deficient vaginal microbiota, is associated with adverse health outcomes. BV often recurs after standard antibiotic therapy in part because antibiotics promote microbiota dominance by Lactobacillus iners instead of Lactobacillus crispatus, which has more beneficial health associations. Strategies to promote L. crispatus and inhibit L. iners are thus needed. We show that oleic acid (OA) and similar long-chain fatty acids simultaneously inhibit L. iners and enhance L. crispatus growth. These phenotypes require OA-inducible genes conserved in L. crispatus and related lactobacilli, including an oleate hydratase (ohyA) and putative fatty acid efflux pump (farE). FarE mediates OA resistance, while OhyA is robustly active in the vaginal microbiota and enhances bacterial fitness by biochemically sequestering OA in a derivative form only ohyA-harboring organisms can exploit. OA promotes L. crispatus dominance more effectively than antibiotics in an in vitro BV model, suggesting a metabolite-based treatment approach.
Keywords: Lactobacillus; bacterial vaginosis; female genital tract; metabolism; vaginal microbiome; women’s health.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.Z., S.M.B., P.C.B., and D.S.K. are co-inventors on a patent related to this work. P.C.B. serves as a consultant and equity holder of companies in the microfluidics and life sciences industries, including 10x Genomics, GALT/Isolation Bio, Celsius Therapeutics, Next Gen Diagnostics, Cache DNA, Concerto Biosciences, Amber Bio, Stately, Ramona Optics, and Bifrost Biosystems. D.S.K. serves as an equity holder of Day Zero Diagnostics.
Figures







Update of
-
Vaginal Lactobacillus fatty acid response mechanisms reveal a novel strategy for bacterial vaginosis treatment.bioRxiv [Preprint]. 2023 Dec 30:2023.12.30.573720. doi: 10.1101/2023.12.30.573720. bioRxiv. 2023. Update in: Cell. 2024 Sep 19;187(19):5413-5430.e29. doi: 10.1016/j.cell.2024.07.029. PMID: 38234804 Free PMC article. Updated. Preprint.
References
-
- Moreno I., Codoñer F.M., Vilella F., Valbuena D., Martinez-Blanch J.F., Jimenez-Almazán J., Alonso R., Alamá P., Remohí J., Pellicer A., et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. - DOI - PubMed
-
- Gaudoin M., Rekha P., Morris A., Lynch J., Acharya U. Bacterial vaginosis and past chlamydial infection are strongly and independently associated with tubal infertility but do not affect in vitro fertilization success rates. Fertil. Steril. 1999;72:730–732. doi: 10.1016/s0015-0282(99)00310-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

