Steamed hay and alfalfa pellets for the management of severe equine asthma
- PMID: 39164027
- PMCID: PMC11982419
- DOI: 10.1111/evj.14209
Steamed hay and alfalfa pellets for the management of severe equine asthma
Abstract
Background: Steaming hay significantly reduces respirable particles and provides a palatable alternative to dry hay for horses with asthma, but there are few prospective studies demonstrating the clinical efficacy of steamed hay.
Objectives: To compare the efficacy of alfalfa pellets and steamed hay in improving lung function and inflammation of horses with severe asthma (SEA).
Study design: Controlled crossover study.
Methods: Ten horses with SEA were enrolled and nine completed the study. Horses were housed indoors and fed hay. Once in exacerbation, they were fed pellets and steamed hay for 4 weeks, in a crossover design. Weighted clinical scores and lung function were recorded weekly. Bronchoalveolar lavage fluid (BALF) cytology and mucus scores were recorded before and after each diet.
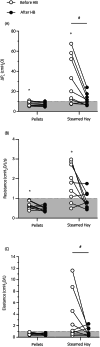
Results: Based on linear mixed model (LMM) analysis, weighted clinical scores significantly improved over time (p < 0.001, no diet effect or time-diet interactions). With pellets, weighted clinical scores decreased from (median (interquartile range)) 13 (5.5) to 2 (1.5), while with steamed hay, they decreased from 10 (9.5) to 6 (8.5). With pellets, lung resistance decreased significantly from a baseline of (mean (SD)) 2.62 (0.77) cmH2O/L/s to 1.17 (0.66), 0.79 (0.54), 0.70 (0.20), 0.62 (0.18) on Weeks 1-4, respectively (LMM with post hoc tests, p < 0.001). With steamed hay, the resistance decreased significantly from a baseline of 2.34 (0.93) cmH2O/L/s to 1.38 (0.49) and 1.51 (0.66) on Weeks 1 and 2, respectively (p < 0.03). Neutrophils BALF decreased significantly with both diets (pellets: 40.2 (24.4) to 20.1 (11.0) %; steamed hay 30.9 (20.2) to 25.7 (17.6) %; LMM, p = 0.006).
Main limitations: A small number of horses in a research setting. Dust was not measured in the stalls.
Conclusions: Clinical scores, lung function and BALF inflammation of horses with SEA improved with steamed hay and pellets, but the effect on lung function was more pronounced with pellets.
Keywords: dust; environment; heaves; horse; mucus; recurrent airway obstruction.
© 2024 The Author(s). Equine Veterinary Journal published by John Wiley & Sons Ltd on behalf of EVJ Ltd.
Conflict of interest statement
Mathilde Leclère has received honoraria from Boehringer Ingelheim, unrelated to this study. Other authors declare no conflict of intererst.
Figures





Similar articles
-
Effects of soaked hay on lung function and airway inflammation in horses with severe asthma.J Vet Intern Med. 2024 Jan-Feb;38(1):469-476. doi: 10.1111/jvim.16919. Epub 2023 Nov 6. J Vet Intern Med. 2024. PMID: 37930110 Free PMC article.
-
The influence of hay steaming on clinical signs and airway immune response in severe asthmatic horses.BMC Vet Res. 2018 Nov 15;14(1):345. doi: 10.1186/s12917-018-1636-4. BMC Vet Res. 2018. PMID: 30442129 Free PMC article.
-
Effects of low-dust forages on dust exposure, airway cytology, and plasma omega-3 concentrations in Thoroughbred racehorses: A randomized clinical trial.J Vet Intern Med. 2023 Jan;37(1):338-348. doi: 10.1111/jvim.16598. Epub 2022 Dec 7. J Vet Intern Med. 2023. PMID: 36478588 Free PMC article.
-
Dust exposure and pulmonary inflammation in Standardbred racehorses fed dry hay or haylage: A pilot study.Vet J. 2021 May;271:105654. doi: 10.1016/j.tvjl.2021.105654. Epub 2021 Mar 13. Vet J. 2021. PMID: 33840486
-
Towards personalized medicine for the treatment of equine asthma.Vet J. 2024 Jun;305:106125. doi: 10.1016/j.tvjl.2024.106125. Epub 2024 May 3. Vet J. 2024. PMID: 38704018 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

