Clinical value of guideline recommended molecular targets and genome targeted cancer therapies: cross sectional study
- PMID: 39164034
- PMCID: PMC11333991
- DOI: 10.1136/bmj-2023-079126
Clinical value of guideline recommended molecular targets and genome targeted cancer therapies: cross sectional study
Abstract
Objective: To assess the clinical benefit and actionability of molecular targets for genome targeted cancer drugs recommended for clinical practice by the National Comprehensive Cancer Network (NCCN).
Design: Cross sectional study.
Participants/setting: Genome targeted cancer drugs recommended by NCCN guidelines in the advanced setting.
Main outcome measures: Molecular target actionability was assessed using the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT). Clinical benefit of genome targeted oncology therapies was evaluated using the ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS). Molecular targets at ESCAT category level I associated with studies showing substantial clinical benefit by ESMO-MCBS (grades 4-5) were designated as high benefit, and those linked to studies achieving an ESMO-MCBS grade of 3 were categorized as being of promising but unproven benefit.
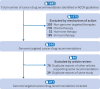
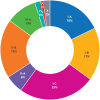
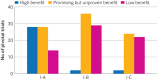
Results: 411 recommendations related to 74 genome targeted drugs targeting 50 driver alterations were examined. Most recommendations (346/411; 84%) were associated with clinical trials of various phases, but 16% (65/411) relied on only case reports or pre-clinical studies. However, clinical trials mostly comprised phase I or phase II (271/346; 78%), single arm (262/346; 76%) studies. The primary endpoint assessed in most trials was overall response rate (271/346; 78%) rather than survival. ESCAT tier I targetability encompassed 60% (246/411) of target recommendations, 35% (142/411) were classified as tier II or III, and 6% (23/411) had their relevance yet to be determined (tiers IV to X). When ESMO-MCBS was applied to 267 scorable trials, only 12% (32/267) showed substantial clinical benefit (grades 4-5) and 45% (121/267) were grade 3. When both frameworks were combined, 12% (32/267) of trials supported a determination of high benefit and 33% (88/267) indicated promising but unproven benefit. Of the 118 interventions endorsed by NCCN authors as preferred, 62 (53%) applied to treatments with high or promising but unproven benefit.
Conclusion: According to the ESCAT and ESMO-MCBS frameworks, about one eighth of genome based treatments for solid cancer were rated as likely to offer a high benefit to patients, whereas around a third were identified as offering a promising but unproven substantial benefit. Ensuring that NCCN recommendations are aligned with expected clinical benefits is crucial for promoting informed, evidence based, genomic guided treatment decisions.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from Kaiser Permanent Institute for Health Policy and Arnold Ventures for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Swain SM, Miles D, Kim SB, et al. CLEOPATRA study group . Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519-30. 10.1016/S1470-2045(19)30863-0 - DOI - PubMed
-
- My Cancer Genome. Biomarkers. https://www.mycancergenome.org/content/biomarkers/#biomarker_type=Geneti....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
