Small molecule valproic acid enhances ventral patterning of human neural tube organoids by regulating Wnt and Shh signalling
- PMID: 39164046
- PMCID: PMC11693559
- DOI: 10.1111/cpr.13737
Small molecule valproic acid enhances ventral patterning of human neural tube organoids by regulating Wnt and Shh signalling
Abstract
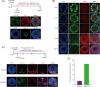
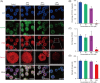
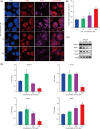
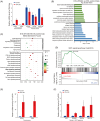
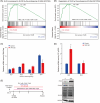
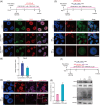
Valproic acid (VPA), a clinically approved small molecule, has been reported to activate Wnt signalling that is critical for dorsal-ventral (DV) patterning of neural tube. However, little is known about the impact of VPA on DV patterning process. Here, we show that even though VPA has a negative impact on the early formation of human neural tube organoids (hNTOs), it significantly enhances the efficiency of ventrally patterned hNTOs, when VPA is added during the entire differentiation process. RNA sequencing and RT-qPCR analysis demonstrates VPA activates endogenous Wnt signalling in hNTOs. Surprisingly, transcriptome analysis also identifies upregulation of genes for degradation of GLI2 and GLI3 proteins, whose truncated fragment are transcriptional repressors of Shh signalling. The Western-blot analysis confirms the increase of GLI3R proteins after VPA treatment. Thus, VPA might enhance ventral patterning of hNTOs through both activating Wnt, which can antagonise Shh signalling by inducing GLI3 expression, and/or inhibiting Shh signalling by inducing GLI protein degradation. We further obtain results to show that VPA still increases patterning efficiency of hNTOs with a weak influence on their early formation when the initiation time of VPA is delayed and its duration is reduced. Taken together, this study demonstrates that VPA enhances the generation of more reproducible hNTOs with ventral patterning, opening the avenues for the applications of hNTOs in developmental biology and regenerative medicine.
© 2024 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

