Pharmacokinetics of Extended-release Buprenorphine and Clinical Efficacy for Postoperative Pain Management in the Domestic Ferret (Mustela putorius furo)
- PMID: 39164072
- PMCID: PMC11467869
- DOI: 10.30802/AALAS-JAALAS-24-000011
Pharmacokinetics of Extended-release Buprenorphine and Clinical Efficacy for Postoperative Pain Management in the Domestic Ferret (Mustela putorius furo)
Abstract
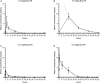
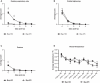
Buprenorphine hydrochloride (Bup-HCl) is a common injectable opioid analgesic. In ferrets, Bup-HCl must be administered every 8 to 12 h to maintain clinical efficacy. Extended-release analgesics offer multiple advantages, including reduced handling and injection frequency, improved compliance, and increased protection from end-of-dose failure. Although efficacy of extended-release buprenorphine formulations has been demonstrated in other species, their use in the domestic ferret has not been investigated. In this study, we evaluated the pharmacokinetics of a compounded polymeric formulation of buprenorphine (Bup-ER) and a pharmaceutical-grade, FDA-indexed liposomal suspension (Bup-XR). Two doses each of Bup-ER (0.12 and 0.2 mg/kg) and Bup-XR (0.2 and 0.6 mg/kg SC) were administered to young adult female ferrets and plasma concentrations were measured between 0 and 96 h (n = 4 animals per timepoint). All doses of both drugs achieved therapeutic plasma levels by 30 min. Furthermore, high-dose Bup-XR maintained therapeutic levels for 72 h, followed by high-dose Bup-ER (less than 48 h), low-dose Bup-XR (24 h), and low-dose Bup-ER (less than 24 h). In this study, we also developed a pain scoring system and utilized this to compare analgesic efficacy between single high-dose Bup-XR (0.6 mg/kg SC) and a standard postoperative course of Bup-HCl (0.02 mg/kg SC every 10 to 12 h for 8 doses) after ovariohysterectomy. Ferrets receiving Bup-XR had significantly lower respiratory rate and posture scores in the first 24 h postoperatively than did those that received Bup-HCl and were less likely to react to palpation of the surgical incision. Of note, ferrets that received high-dose Bup-ER had a significantly higher incidence of injection site reactions than ferrets that received Bup-HCl (P = 0.0137). This study demonstrates that a single dose of Bup-XR (0.6 mg/kg SC) is a safe and effective analgesic in female ferrets, with a duration of action up to 72 h and minimal side effects, offering a refinement to analgesia in this species.
Conflict of interest statement
Fidelis Animal Health provided the supply of Bup-XR for this study. The authors have no conflicts of interest to declare.
Figures




References
-
- American Veterinary Medical Association. [Internet]. 2018. U.S. pet ownership statistics. [Cited 12 June 2023]. Available at: https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership....
LinkOut - more resources
Full Text Sources

