Gut epithelial electrical cues drive differential localization of enterobacteria
- PMID: 39164392
- PMCID: PMC11445056
- DOI: 10.1038/s41564-024-01778-8
Gut epithelial electrical cues drive differential localization of enterobacteria
Abstract
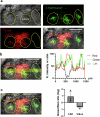
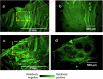
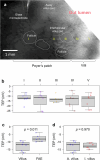
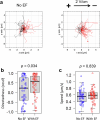
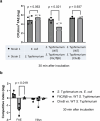
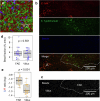
Salmonella translocate to the gut epithelium via microfold cells lining the follicle-associated epithelium (FAE). How Salmonella localize to the FAE is not well characterized. Here we use live imaging and competitive assays between wild-type and chemotaxis-deficient mutants to show that Salmonella enterica serotype Typhimurium (S. Typhimurium) localize to the FAE independently of chemotaxis in an ex vivo mouse caecum infection model. Electrical recordings revealed polarized FAE with sustained outward current and small transepithelial potential, while the surrounding villus is depolarized with inward current and large transepithelial potential. The distinct electrical potentials attracted S. Typhimurium to the FAE while Escherichia coli (E. coli) localized to the villi, through a process called galvanotaxis. Chloride flux involving the cystic fibrosis transmembrane conductance regulator (CFTR) generated the ionic currents around the FAE. Pharmacological inhibition of CFTR decreased S. Typhimurium FAE localization but increased E. coli recruitment. Altogether, our findings demonstrate that bioelectric cues contribute to S. Typhimurium targeting of specific gut epithelial locations, with potential implications for other enteric bacterial infections.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures










References
-
- Centers for Disease Control and Prevention. Preliminary FoodNet Data on the incidence of infection with pathogens transmitted commonly through food–10 States, 2008. Morb. Mortal. Wkly Rep. 58, 333–337 (2009). - PubMed
-
- Lynch, M. F., Tauxe, R. V. & Hedberg, C. W. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect.137, 307–315 (2009). - PubMed
MeSH terms
Substances
Grants and funding
- R21 AI156409/AI/NIAID NIH HHS/United States
- R01 EY019101/EY/NEI NIH HHS/United States
- HR001119S0027/United States Department of Defense | Defense Advanced Research Projects Agency (DARPA)
- 1R21AI156409/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- P30 EY012576/EY/NEI NIH HHS/United States

