Efficacy and safety of bosutinib in previously treated patients with chronic myeloid leukemia: final results from the BYOND trial
- PMID: 39164407
- PMCID: PMC11436368
- DOI: 10.1038/s41375-024-02372-x
Efficacy and safety of bosutinib in previously treated patients with chronic myeloid leukemia: final results from the BYOND trial
Abstract
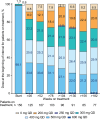
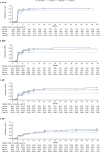
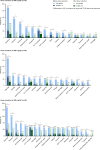
This final analysis from the phase 4 BYOND trial reports outcomes with bosutinib in patients with previously treated chronic myeloid leukemia (CML); 163 patients with CML resistant/intolerant to previous tyrosine kinase inhibitors received bosutinib (starting dose: 500 mg QD). At study completion (median follow-up, 47.8 months), 48.1% (n = 75/156) of patients with Philadelphia chromosome-positive chronic phase CML were still receiving treatment. Among evaluable patients, 71.8% (95% CI, 63.9-78.9) and 59.7% (95% CI, 51.4-67.7) attained or maintained major molecular response (MMR) and molecular response (MR)4, respectively, at any time on treatment. The majority of patients achieved a deeper molecular response relative to baseline while on bosutinib. Kaplan-Meier probabilities (95% CI) of maintaining MMR and MR4 at 36 months were 87.2% (78.0-92.7) and 80.7% (69.4-88.1), respectively. At 48 months, the Kaplan-Meier overall survival rate was 88.3% (95% CI, 81.8-92.6); there were 17 deaths, including 2 that were considered CML related. Long-term adverse events (AEs) were consistent with the known safety profile of bosutinib, and no new safety issues were identified. The management of AEs through dose reduction maintained efficacy while improving tolerability. These results support the use of bosutinib in patients with previously treated CML.ClinicalTrials.gov, NCT02228382.
© 2024. The Author(s).
Conflict of interest statement
CG-P: consultancy (Bristol Myers Squibb), honoraria and research funding (Pfizer). THB: research funding (Bristol Myers Squibb, Novartis, Pfizer, Repeat Diagnostic); honoraria (Janssen, Novartis, Pfizer, Takepart Media); consultancy (Pfizer). EA: consultancy and honoraria (Bristol Myers Squibb, Incyte, Novartis, Pfizer). KRK: consultancy (Amgen, AstraZeneca, Denovo Biopharma, Sanofi-Aventis, Takeda, Verastem); equity holder (Agios, Berkley Lights); Speakers Bureau (Bayer, Celgene, Epizyme, Gilead, Janssen, Karyopharm, Novartis, Pharmacyclics). VGO: research funding and lectures (Pfizer); consultancy (Pfizer, Novartis, Ascentage, Terns); honoraria (OncLive). VG-G: research funding (Pfizer, Novartis, BMS, Incyte); consultancy (Bristol Myers Squibb, Incyte, Novartis). HH-H: research funding and lectures (AOP, Incyte, Pfizer).TE: research support from Bristol Myers Squibb, Incyte, Novartis, and Pfizer. EL, SP, GL, AV: employee (Pfizer); equity holder (Pfizer). FJG: consultancy (Novartis). AH: research funding (Bristol Myers Squibb, Incyte, Novartis, Pfizer).
Figures
References
-
- Pfizer Inc. Bosulif (bosutinb) prescribing information. 2012. https://labeling.pfizer.com/showlabeling.aspx?id=884. Accessed 5 Sep 2023.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

