CCKBR+ cancer cells contribute to the intratumor heterogeneity of gastric cancer and confer sensitivity to FOXO inhibition
- PMID: 39164456
- PMCID: PMC11445462
- DOI: 10.1038/s41418-024-01360-z
CCKBR+ cancer cells contribute to the intratumor heterogeneity of gastric cancer and confer sensitivity to FOXO inhibition
Abstract
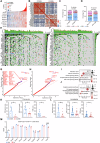
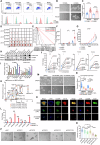
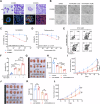
The existence of heterogeneity has plunged cancer treatment into a challenging dilemma. We profiled malignant epithelial cells from 5 gastric adenocarcinoma patients through single-cell sequencing (scRNA-seq) analysis, demonstrating the heterogeneity of gastric adenocarcinoma (GA), and identified the CCKBR+ stem cell-like cancer cells associated poorly differentiated and worse prognosis. We further conducted targeted analysis using single-cell transcriptome libraries, including 40 samples, to confirm these screening results. In addition, we revealed that FOXOs are involved in the progression and development of CCKBR+ gastric adenocarcinoma. Inhibited the expression of FOXOs and disrupting cancer cell stemness reduce the CCKBR+ GA organoid formation and impede tumor progression. Mechanically, CUT&Tag sequencing and Lectin pulldown revealed that FOXOs can activate ST3GAL3/4/5 as well as ST6GALNAC6, promoting elevated sialyation levels in CCKBR+ tumor cells. This FOXO-sialyltransferase axis contributes to the maintenance of homeostasis and the growth of CCKBR+ tumor cells. This insight provides novel perspectives for developing targeted therapeutic strategies aimed at the treating CCKBR associated gastric cancer.
© 2024. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
-
- Imamura K, Yao K, Nimura S, Tanabe H, Kanemitsu T, Miyaoka M, et al. Characteristic endoscopic findings of gastric adenocarcinoma of fundic-gland mucosa type. Gastric Cancer. 2021;24:1307–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

