Retinoic acid induces human gastruloids with posterior embryo-like structures
- PMID: 39164488
- PMCID: PMC11469962
- DOI: 10.1038/s41556-024-01487-8
Retinoic acid induces human gastruloids with posterior embryo-like structures
Abstract
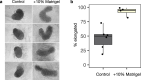
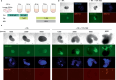
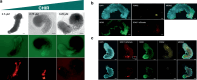
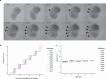
Gastruloids are a powerful in vitro model of early human development. However, although elongated and composed of all three germ layers, human gastruloids do not morphologically resemble post-implantation human embryos. Here we show that an early pulse of retinoic acid (RA), together with later Matrigel, robustly induces human gastruloids with posterior embryo-like morphological structures, including a neural tube flanked by segmented somites and diverse cell types, including neural crest, neural progenitors, renal progenitors and myocytes. Through in silico staging based on single-cell RNA sequencing, we find that human RA-gastruloids progress further than other human or mouse embryo models, aligning to E9.5 mouse and CS11 cynomolgus monkey embryos. We leverage chemical and genetic perturbations of RA-gastruloids to confirm that WNT and BMP signalling regulate somite formation and neural tube length in the human context, while transcription factors TBX6 and PAX3 underpin presomitic mesoderm and neural crest, respectively. Looking forward, RA-gastruloids are a robust, scalable model for decoding early human embryogenesis.
© 2024. The Author(s).
Conflict of interest statement
J.S. is a scientific advisory board member, consultant and/or co-founder of Prime Medicine, Cajal Neuroscience, Guardant Health, Maze Therapeutics, Camp4 Therapeutics, Phase Genomics, Adaptive Biotechnologies, Scale Biosciences, Sixth Street Capital, Pacific Biosciences and Somite Therapeutics. The other authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

