Clinical efficacy and immune response of neoadjuvant camrelizumab plus chemotherapy in resectable locally advanced oesophageal squamous cell carcinoma: a phase 2 trial
- PMID: 39164491
- PMCID: PMC11442672
- DOI: 10.1038/s41416-024-02805-5
Clinical efficacy and immune response of neoadjuvant camrelizumab plus chemotherapy in resectable locally advanced oesophageal squamous cell carcinoma: a phase 2 trial
Abstract
Background: Neoadjuvant immunotherapy is under intensive investigation for esophageal squamous cell carcinoma (ESCC). This study assesses the efficacy and immune response of neoadjuvant immunochemotherapy (nICT) in ESCC.
Methods: In this phase II trial (ChiCTR2100045722), locally advanced ESCC patients receiving nICT were enrolled. The primary endpoint was the pathological complete response (pCR) rate. Multiplexed immunofluorescence, RNA-seq and TCR-seq were conducted to explore the immune response underlying nICT.
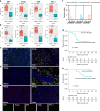
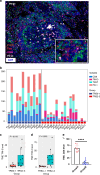
Results: Totally 42 patients were enrolled, achieving a 27.0% pCR rate. The 1-year, 2-year DFS and OS rates were 89.2%, 64.4% and 97.3%, 89.2%, respectively. RNA-seq analysis highlighted T-cell activation as the most significantly enriched pathway. The tumour immune microenvironment (TIME) was characterised by high CD4, CD8, Foxp3, and PD-L1 levels, associating with better pathological regression (TRS0/1). TIME was categorised into immune-infiltrating, immune-tolerant, and immune-desert types. Notably, the immune-infiltrating type and tertiary lymphoid structures correlated with improved outcomes. In the context of nICT, TIM-3 negatively influenced treatment efficacy, while elevated TIGIT/PD-1 expression post-nICT correlated positively with CD8+ T cell levels. TCR-seq identified three TCR rearrangements, underscoring the specificity of T-cell responses.
Conclusions: Neoadjuvant camrelizumab plus chemotherapy is effective for locally advanced, resectable ESCC, eliciting profound immune response that closely associated with clinical outcomes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- van Hagen P, Hulshof M, van Lanschot J, Steyerberg E, van Berge Henegouwen M, Wijnhoven B, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. - PubMed
-
- Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–803. - PMC - PubMed
-
- Schachter J, Ribas A, Long G, Arance A, Grob J, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–62. - PubMed
-
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

